(C) 2010 Andrea K. Walker. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The New World species of Eurytenes Foerster sensu stricto (Hymenoptera: Braconidae, Opiinae) are revised, and a key to these species is presented. Four new species are described: Eurytenes (Eurytenes) dichromus sp. n. from Texas, Eurytenes (Eurytenes) microsomus sp. n. from Texas, Eurytenes (Eurytenes) pachycephalus sp. n. from Mexico, and Eurytenes (Eurytenes) ormenus sp. n. from Mexico. Eurytenes abnormis (Wesmael) is redescribed for comparison, and its host records are reviewed.
Parasitoid, endoparasite, Agromyzidae, pterostigma
Eurytenes was originally described by
In addition to the type species, three other species are currently included in Eurytenes s. str.: Eurytenes orientalis Fischer, 1966, Eurytenes cratospilum Chen & Weng, 2005, and Eurytenes basinervis Wu & Chen, 2006. Eurytenes abnormis is Holarctic (
Nearly all of the material used in this revision is from the Texas A&M University Insect Collection (TAMU). Material for comparison was obtained from or examined at the following institutions: American Entomological Institute (AEI), Gainesville, FL, USA; Canadian National Collection, Ottawa, Canada; Institut Royal des Sciences Naturelles de Belgique, Brussels, Belgium; Naturhistorisches Museum, Vienna, Austria (NHMW); U. S. National Museum of Natural History, Washington, D. C. (USNM). The newly described species were compared with several Old World specimens, including Eurytenes abnormis from eight European localities, the holotype of Eurytenes orientalis, two undetermined specimens from Taiwan, and one undetermined specimen from the Kurile Islands.
Descriptive terminology largely follows
In the material examined sections, label data are presented in a uniform format for specimens other than the holotypes of new species. For holotypes of newly described species, data are recorded exactly as given on specimen labels, with square brackets for additional data not on the labels.
Images were acquired digitally using Syncroscopy’s
Auto-Montage Pro 5.01.0005 (Copyright Synoptics Ltd.) and PictureFrame
(TM) Application 2.3 in combination with a ProgRes 3008 digital camera
mounted on a Leica MZ APO dissecting microscope. All images were further
processed using Adobe Photoshop® CS5. Images are stored in mx, a
web-based content management system that facilitates data management and
dissemination for taxonomic and phylogenetic works (e. g.
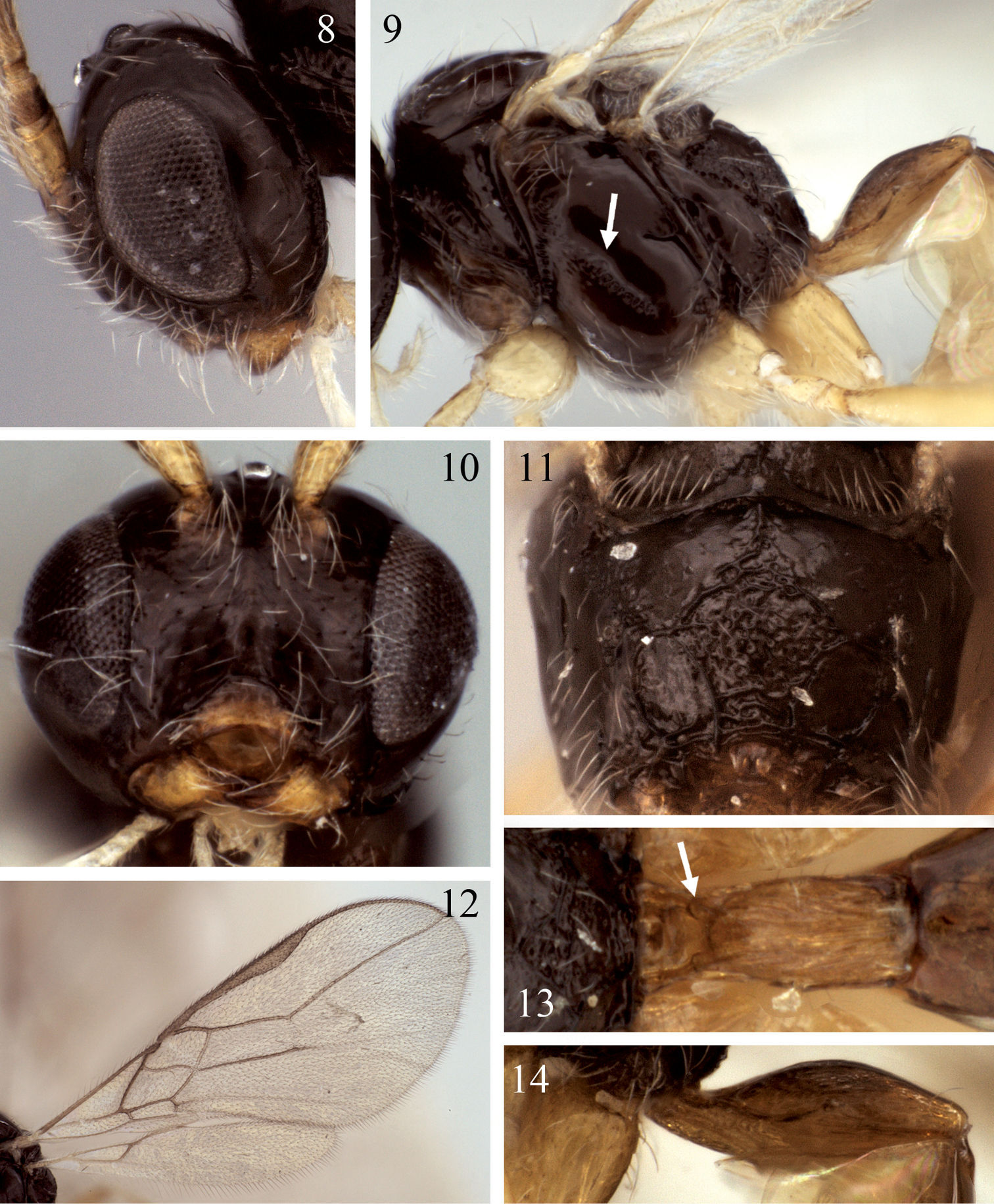
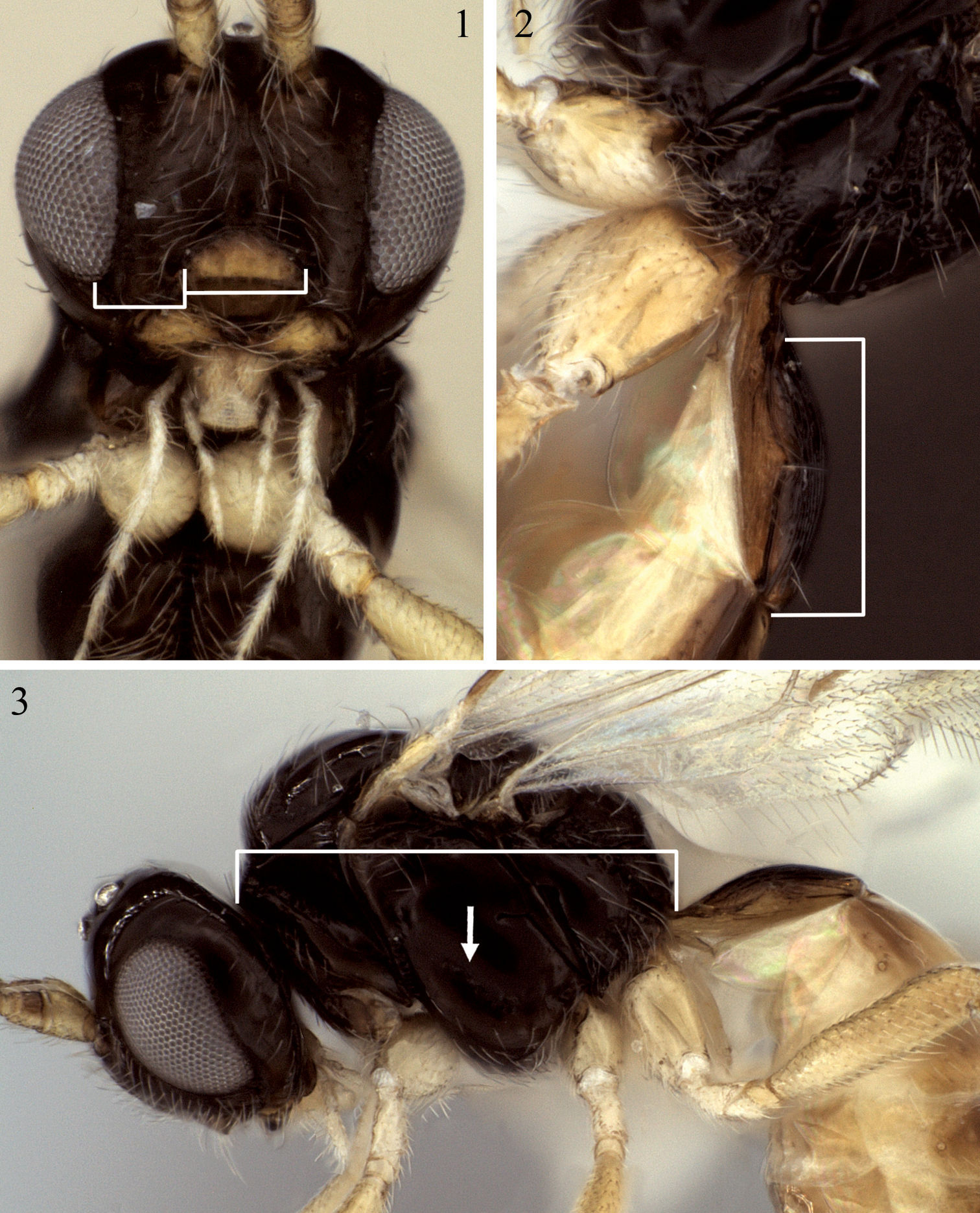
1 Eurytenes microsomus sp. n., face, bars = clypeal measurements 2 Eurytenes dichromus sp. n., petiole, lateral view, bar = length measurement 3 Eurytenes microsomus, lateral view, arrow = precoxal sulcus, bar = mesosomal length.
Head. Antenna filiform, longer than body. Frons, vertex, and temple smooth, shiny; frons bare, vertex and upper temple nearly so. Labrum exposed. Clypeus weakly to distinctly protruding in profile. Malar sulcus deeply impressed. Mandible gradually to somewhat more abruptly widening from apex to base, carinate ventrally over most of basal half, never with distinct basal tooth as in Opius s. str. Maxillary palp longer than head, reaching mid coxa. Occipital carina present laterally, extending dorsal-medially at least to level of inner eye margin, broadly absent mid-dorsally; widely separated from hypostomal carina at base of mandible.
Mesosoma. Pronotum dorsally with narrow, transverse, crenulate sulcus extending continuously along lateral pronotum to ventral corner; weak to deep median pit interrupting sulcus dorsally. Mesoscutum in profile with anterior declivity very slightly concave, nearly vertical; notaulus present on anterior portion of mesoscutal disc, angled laterally at anterior end, laterally-directed portion carinate along anterior margin; notaulus continuous with weakly to distinctly crenulate impression bordering lateral mesoscutal margin, the impression extending posteriorly at least to level of tegula; midpit of mesoscutum well-developed, discrete. Scutellar sulcus (Fig. 31) narrow, though not exceptionally so, 3–4 times wider than mid-length, crenulate, with numerous closely-spaced ridges. Mesopleuron smooth, shiny, posterior margin not crenulate; precoxal sulcus distinctly impressed, crenulate. Propodeum with large median areola, variously obscured by sculpture.
Wings. Slightly more than twice as long as wide. Stigma long, very narrow, nearly parallel-sided basally, widening distally; thickest part of stigma twice maximum width of proximal half. Radial cross-vein (r) thickened, weakly to strongly bowed anteriorly, arising from extreme base of stigma, nearly in line with 3RSa; RS+M weakly to distinctly sinuate; second submarginal cell nearly parallel-sided, not or only very weakly converging distally; m-cu nearly always postfurcal, entering second submarginal cell; 2CUa distinct, shorter than 2cu-a. Hind wing with both RS and M distinct nearly to wing margin, usually nebulous: very weakly pigmented; m-cu varying from indistinct in smaller individuals to present in larger individuals as a weakly pigmented impression extending more than half way to wing margin.
Legs. Hind femur slender, dorsal surface uneven, somewhat bilobed.
Metasoma. Petiole with distinct dorsope; dorsal and lateral surface densely striate to strigose, largely obscuring dorsal carinae except at base; spiracle of T1 located at or slightly posteriad mid-length; sternite short but distinct, extending 0.4–0.5 distance between base of T1 and spiracle. Metasoma posteriad petiole pyriform, unsculptured, with row of setae evenly spaced on posterior margin of tergites; T2 spiracle laterally displaced. Ovipositor nearly straight, without dorsal node; ovipositor sheath setose throughout.
Agromyzidae. See comments under Eurytenes abnormis, the only species with host records.
| 1 | Hind femur almost entirely dark brown (Fig. 5); petiole entirely dark brown to black, more than twice longer than apical width. Mexico | Eurytenes ormenus |
| – | Hind femur yellow, sometimes slightly infuscated distally; petiole variable | 2 |
| 2 | Gena broad (Fig. 28); petiole uniformly dark brown to black (Fig. 29). Mexico | Eurytenes pachycephalus |
| – | Gena narrow (Figs 8, 15, 24); petiole partly (Figs 3, 17) to entirely (Fig. 13) yellow | 3 |
| 3 | Petiole yellow (Fig. 13); clypeus somewhat chevron shaped (Fig. 10) | Eurytenes abnormis |
| – | Petiole mostly dark brown to black dorsally (Fig. 17), yellow laterally (Fig. 3); clypeus truncate or nearly so ventrally, broader and more nearly semi-circular (Figs 1, 18) | 4 |
| 4 | Antenna with 31–35 flagellomeres; clypeus obviously infuscate dorsally (Fig. 18) | Eurytenes dichromus |
| – | Antenna with 29–31 flagellomeres; clypeus barely infuscate dorsally (Fig. 1) | Eurytenes microsomus |
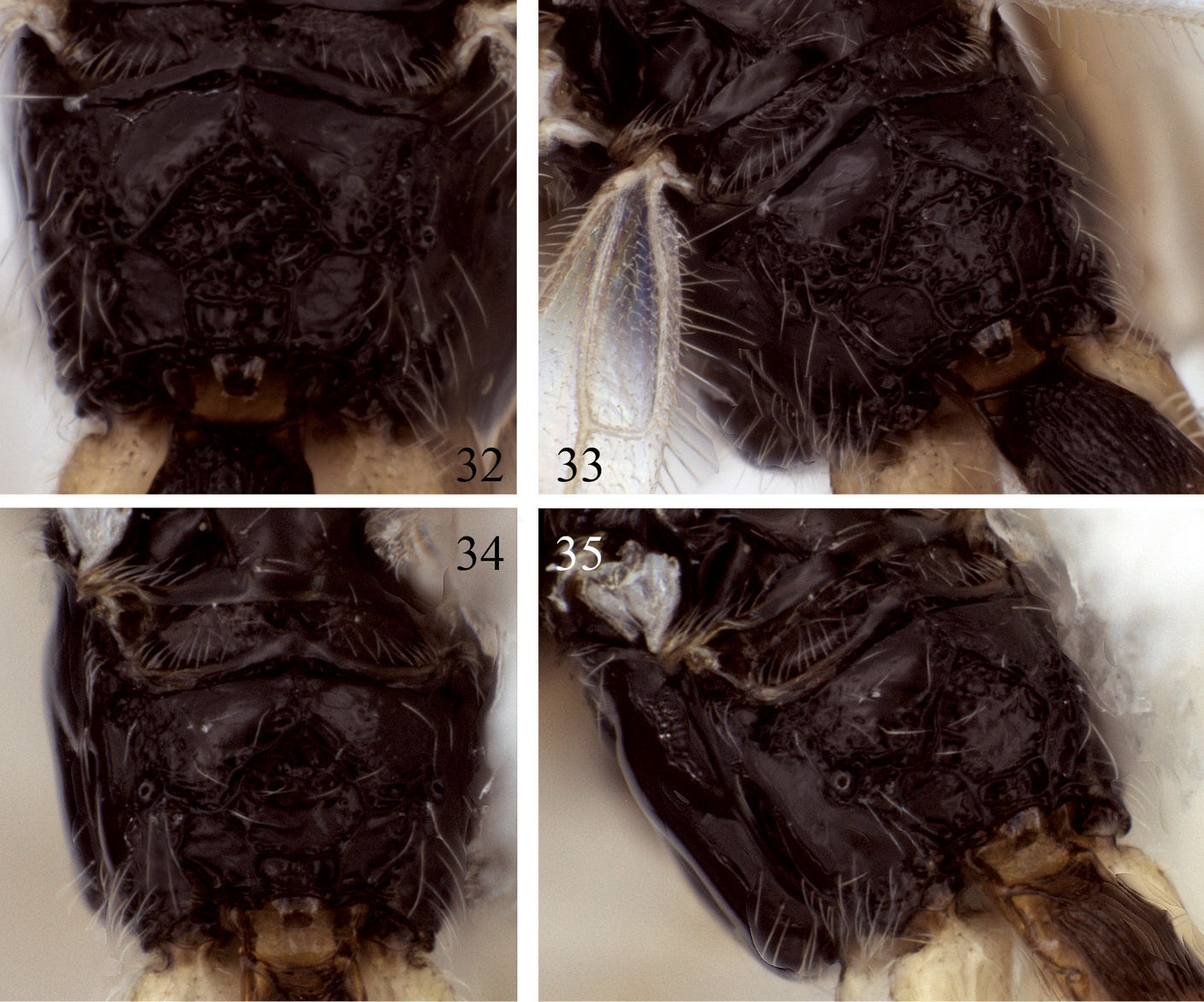
Figs 4, 8–14, 31
♀♂, BELGIUM: vicinity of Brussels, v.18??, C. Wesmael (syntypes, Brussels). 1 ♀, ENGLAND: Sheffield, 16–17.viii.1991, R. Wharton (TAMU). 1 ♀, HUNGARY: Protected forest, 13.vi.1974, Hámoriné & Marótiné (TAMU). IRELAND: 3 ♀, Co. Sligo, Trawalua, 10.vii.1936 & 2.viii.1938, A.W.Stelfox (USNM); 1 ♀, Co. Wicklow, Manor Kilbride, 19.vii.1950, A.W. Stelfox (USNM).
Eurytenes abnormis is most readily recognized by the pale coloration of the petiole and metasoma and is further distinguished from the four North American species described below by the narrower, more ventrally concave clypeus (Fig. 10). The petiole is narrower than in Eurytenes dichromus, sp. n. and Eurytenes microsomus, sp. n. and is thus more similar in shape to the darker Mexican species described below.
(♀). Length of body: 1.9–2.4 mm (m=2.2), length of fore wing 2.3–3.0 mm (m=2.7).
Head. 27–31 flagellomeres; first flagellomere length 4.0–5.0 × width (m=4.4), fifteenth flagellomere length 2.0–3.5 × width (m=2.9), fifth from last flagellomere length 2.2–3.0 × width (m=2.7). Face 1.4–1.7 (m=1.5) × wider than high. Clypeus broadly chevron-shaped, with ventral margin concave; 2.0–3.0 (m=2.5) × wider than high; 1.3–1.8 (m=1.5) × wider than distance between clypeus and eye. Mandible distinctly expanded over basal 0.3, with flange-like ventral carina. Gena relatively narrow (Fig. 8). Occipital carina dorsally extending about 0.6–0.7 × distance from eye to nearest lateral ocellus.
Mesosoma. Posterior-ventral margin of lateral pronotum crenulate for most of length. Precoxal sulcus parallel-sided, narrowly crenulate along most of length, usually weakly impressed anteriorly, often extending very close to anterior margin of mesopleuron; precoxal sulcus inclined at a 35 degree angle. Notaulus distinctly impressed over anterior third of mesoscutal disc, crenulate over anterior 0.2–0.3; with cluster of short setae at rugulose base of anterior declivity; with widely spaced line of 3–5 longer setae extending posteriorly towards but not usually reaching cluster of scattered setae around midpit. Propodeum with median carina present anteriorly, bifurcating near middle to form five-sided median areola over posterior 0.6, surface rugose laterally and posterior-medially, partly obscuring areola, but posterior-lateral fields largely smooth, as in Fig. 11.
Wings. Fore wing r-m tubular and pigmented only at extreme anterior end, otherwise unpigmented, with lateral boundaries often only weakly indicated; (RS+M)b absent, m-cu entering extreme base of second submarginal cell; 3M very weakly pigmented basally in available material, spectral over most of length. Hind wing m-cu varying from indistinct in smaller individuals to present as a spectral impression extending more than half way to wing margin in larger individuals.
Legs. Hind tibia 7.5–9.3 (m=8.7) × longer than maximum width.
Metasoma. Petiole 1.9–2.2 (m=2.0) × longer than apical width. Female ovipositor short but distinctly protruding, about 0.9 × length of mesosoma. Ovipositor sheath about 0.4 × length of mesosoma.
Color. Head and mesosoma dark reddish-brown to black. Scape, pedicel and first flagellomere yellow, antenna quickly darkening distally to dark brown; palps and tegula pale yellow; mandible and petiole tawny (darker yellow). Metasoma posteriad petiole usually bright yellow, sometimes with faint slight butterscotch banding to lighter brown banding. Hind femur and tibia darkening distally, femur transitioning from yellow to dark yellow or yellow-brown, tibia mostly infuscated, tarsi infuscated; legs otherwise yellow. Ovipositor sheath dark brown to black; ovipositor light brown throughout. Wings hyaline.
Eurytenes spp., habitus. 4 Eurytenes abnormis, female 5 Eurytenes ormenus sp. n., female 6 Eurytenes pachycephalus sp. n., male 7 Eurytenes dichromus sp. n., female.
Eurytenes abnormis. 8 Head, lateral view 9 Mesosoma, lateral view, arrow = precoxal sulcus 10 Face 11 Propodeum, posterior view 12 Left fore and hind wing 13 Petiole, dorsal view, arrow = dorsope 14 Petiole, lateral view.
The agromyzid host records found in (
Agromyza albitarsis Meigen: host plant for Eurytenes abnormis has not been recorded previously but since this fly is known to attack several trees in the family Salicaceae, the record may need to be verified;
Agromyza woerzi Groschke: Knautia arvensis (L.) Coult, Caprifoliaceae;
Amauromyza labiatarum (Hendel): Galeopsis tetrahit L., Lamiaceae;
Amauromyza lamii (Kaltenbach): Lamiastrum galeobdolon (L.), Lamiaceae;
Cerodontha angulata (Loew): Carex hirta L., Cyperaceae;
Cerodontha caricivora (Groschke): Carex hirta L., Cyperaceae;
Cerodontha eucaricis Nowakowski: Carex hirta L., Cyperaceae;
Cerodontha flavocingulata (Strobl): Festuca pratensis Huds. (= Lolium pratense) and Holcus lanatus L., Poaceae;
Cerodontha incisa (Meigen): Alopecurus pratensis L. and Phleum pretense L., Poaceae;
Cerodontha iraeos (Robineau-Desvoidy): Iris pseudacorus L., Iridaceae;
Cerodontha pygmaea (Meigen): Dactylis glomerata L. and Deschampsia cespitosa (L.), Poaceae;
Liriomyza balcanica (Strobl): host plant for Eurytenes abnormis has not been previously recorded but this fly is known to attack members of the Euphorbiaceae;
Liriomyza demeijerei Hering: Artemisia vulgaris (L.), Asteraceae;
Liriomyza eupatoriana Spencer: Eupatorium cannabinum L., Asteraceae;
Liriomyza flaveola (Fallén): Festuca pratensis Huds., Poaceae;
Liriomyza scorzonerae Rydén: Scorzonera humilis L., Asteraceae;
Phytoliriomyza variegata (Meigen): host plant for Eurytenes abnormis has not been previously previously but this fly is known to attack members of the Fabaceae;
Phytomyza abdominalis Zetterstedt: Hepatica nobilis Mill., Ranunculaceae;
Phytomyza albimargo Hering: host plant for Eurytenes abnormis has not been recorded previously but this fly is known to attack Anemone in the Ranunculaceae;
Phytomyza fallaciosa Brischke: Ranunculus repens L., Ranunculaceae;
Phytomyza obscura Hendel: Clinopodium vulgare L., Lamiaceae;
Phytomyza pulmonariae Nowakowski: Pulmonaria angustifolia L., Boraginaceae;
Phytomyza senecionis Kaltenbach: Senecio nemorensis fuchsii (=Senecio fuchsii Celak), Asteraceae.
Previously recorded from throughout most of
Europe (specifically Austria, Belgium, Bulgaria, Croatia, England,
Finland, Germany, Hungary, Ireland, Italy, Lithuania, Poland,
western Russia as far as the Urals, and Ukraine). Also recorded from
eastern Palaearctic (Korea and Sakhalin Island), central to eastern
Canada (Ontario, Saskatchewan) and USA (Minnesota, Missouri, North
Dakota, South Carolina). Specific references to individual records can
be found in
In addition to specimens listed in the material
examined section above, two specimens from Poland and one from Germany
(NHMW) were also briefly examined; data for these specimens were
previously recorded by
urn:lsid:zoobank.org:act:FF64FD26-929E-4F40-8DD6-2D22519C6005
Figs 2, 7, 15–21, 30, 32, 33♀ (TAMU): [USA:] TEXAS: Brazos Co. College Station Lick Creek Park 28.iv–11.v.2001 R. Wharton [five lines on a single label].
(TAMU, USNM): 5 ♀, same data as holotype; 5 ♀, same data except 11–21.v.2001; 2 ♀, same data except 15–28.iv.2001; 1 ♀, same data except 1–11.vi.2001; 1 ♀, same data except 6–16.iv.2007, M. Cameron & A. Colvin; 1 ♀, same data except 19.iv-12.v.2009, J. B. Woolley; 1 ♀, Texas, Travis Co., Austin, Longhollow, 10–23.iv.1993. R. Wharton; 1 ♀, Texas, Walker Co., Huntsville, 2.iv.2006, R. Wharton. Additional specimens, not paratypes (TAMU): 2 ♀, Florida, Alachua Co., Hague Dairy, 29 47.311'N, 82 24.880'W, 28.iii.2007, J. Sivinski; 1 ♀, same data except 29 47.328'N, 82 24.969'W, 29.iii.2007; 1 ♀, Texas, Anderson Co., 10 mi SW Elkhart, 5–6.vi.1976, H. R. Burke.
This species is most readily recognized by its broader, bicolored petiole and bicolored, ventrally truncate clypeus. Based on the shape and color pattern of the petiole, as well as the shape of the clypeus, Eurytenes dichromus is most similar to Eurytenes microsomus, sp. n. The propodeum is nearly always more heavily sculptured in Eurytenes dichromus than in Eurytenes microsomus and usually slightly more rugulose posterior-laterally than in Eurytenes abnormis.
(♀). Length of body: 2.4–2.7 mm (m=2.6), length of fore wing 2.9–3.1 mm (m=3.0).
Head. 31–35 flagellomeres; first flagellomere length 2.6–3.3 × width (m=3.0), fifteenth flagellomere length 2.0–2.3 × width (m=2.2), fifth from last flagellomere, length 1.7–2.4 × width (m=2.0). Face 1.6–1.9 (m=1.7) × wider than high. Clypeus semi-ellipsoidal in shape, with ventral margin truncate or nearly so, 2.2–2.8 (m=2.4) × wider than high; 1.3–1.7 (m=1.45) × wider than distance between clypeus and eye. Mandible distinctly expanded over basal 0.3, with flange-like ventral carina. Gena relatively narrow (Fig. 15). Occipital carina extending about 0.3–0.4 × distance from eye to nearest lateral ocellus.
Mesosoma.Posterior-ventral margin of lateral pronotum crenulate for most of length. Precoxal sulcus narrowly crenulate anteriorly, sculptured area broadening posteriorly, usually weakly impressed anteriorly, often extending very close to anterior margin of mesopleuron; precoxal sulcus approximately 45 degrees, inclined more vertically than in Eurytenes abnormis. Notaulus distinctly impressed over anterior third of mesoscutal disc, crenulate over anterior 0.2–0.3; with dense cluster of short setae at rugulose base of anterior declivity extending ventrally to cover most of anterior declivity; with widely spaced line of 3–5 longer setae extending posteriorly towards but not usually reaching cluster of scattered setae around midpit as in Fig. 30. Propodeum with median carina present anteriorly, bifurcating near basal 0.3 to form five-sided median areola over posterior 0.6–0.7, surface extensively rugose laterally and posterior-medially (Figs 32, 33), partly obscuring areola, posterior-lateral fields often completely rugose.
Wings. Fore wing r-m pigmented basally, less commonly over anterior 0.5, otherwise unpigmented, largely tubular, with lateral boundaries usually distinct for most of length; m-cu usually postfurcal, entering base of second submarginal cell, less commonly interstitial; 3M distinctly pigmented, nearly tubular in basal third, gradually weakening and becoming depigmented distally. Hind wing m-cu usually poorly developed, varying from very weakly to distinctly impressed.
Legs. Hind tibia 7.5–9.1 (m=8.25) × longer than maximum width.
Metasoma. Petiole 1.45–1.8 (m=1.6) × longer than apical width. Female ovipositor short, but distinctly protruding, about 0.8 × length of mesosoma. Ovipositor sheath about 0.4 × length of mesosoma.
Color. Head and mesosoma black, with small red-brown spot adjacent eye dorsal-medially near ocelli, face at base of antennae also usually red-brown. Scape and pedicel yellow, flagellomeres dark brown; mandible butterscotch with distal tip infuscated; clypeus infuscated, dark brown dorsally, butterscotch ventrally; palps and tegula yellow. Petiole dark brown dorsally, posterior fifth and ventral-lateral region usually yellow. T2+3 butterscotch medially; T2 and T3 each with a medium brown lateral splotch; T4 and successive tergites each with dark brown transverse banding anteriorly fading to butterscotch posteriorly. Hind tibia pale yellow to whitish over about basal 0.15, remainder infuscated to medium brown, tarsus completely medium brown, legs otherwise yellow to nearly white, with femur and trochantellus often (though not in holotype) darker yellow than coxa and trochanter. Ovipositor sheath dark brown; ovipositor light brown. Wings hyaline.
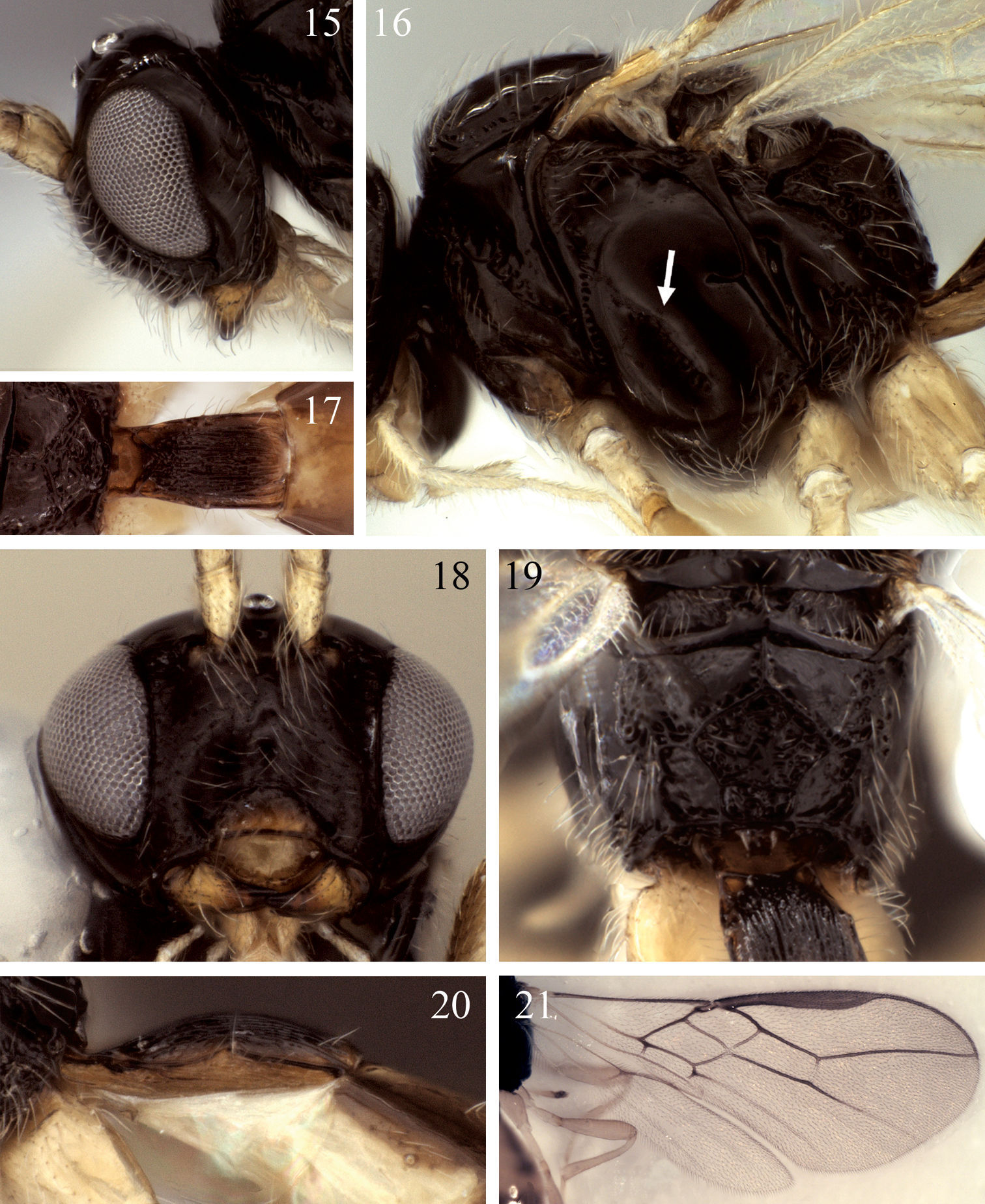
Eurytenes dichromus sp. n. 15 Head, lateral view 16 Mesosoma, lateral view, arrow = precoxal sulcus 17 Petiole, dorsal view 18 Face 19 Propodeum, posterior view 20 Petiole, lateral view 21 Fore and hind wing.
Unknown.
Known only from central Texas.
The name dichromus is derived from Greek: di, two; chromus, color. The name refers to the color of the clypeus.
urn:lsid:zoobank.org:act:7964BF18-1081-49C5-941B-699F6CBDE324
Figs 1, 3, 34, 35♀ (TAMU): [USA:] TX: San Patricio Co. Welder Wildlife Refuge March 23, 1996 R. Wharton [five lines on a single label].
(TAMU): 3 ♀, 3 ♂, same data as holotype; 1 ♂, Texas, Travis Co., Austin, 20.ix.1986, R. Wharton; 1 ♀, same data except 29.iv.1989; 1 ♀, Travis Co., vic. Long Hollow Ck. 30°27'43", -97°52'19", 26.iii.1994, on Quercus buckleyi, M. Quinn, E. Riley, R. Wharton.
This species is nearly identical to Eurytenes dichromus but Eurytenes dichromus is 1.25 × larger. The body is less heavily sculptured than in Eurytenes dichromus, there are fewer flagellomeres, and T2+3 tends to be paler in coloration.
(♀). Length of body: 2.00–2.12 mm (m=2.08), length of fore wing 2.4–2.7 mm (m=2.5).
Head. 29–31 flagellomeres; first flagellomere length 2.3–3.6 × width (m=2.8), fifteenth flagellomere length 2.0–3.0 × width (m=2.3), fifth from last flagellomere length 2.0–2.8 × width (m=2.3). Face 1.4–1.7 (m=1.6) × wider than high. Clypeus more nearly semi-circular in shape, with ventral margin truncate, 2.0–2.5 (m=2.2) × wider than high; 1.5–1.9 (m=1.7) × wider than distance between clypeus and eye. Mandible distinctly expanded over basal 0.3, with flange-like ventral carina. Gena relatively narrow (Fig. 3). Occipital carina extending about 0.3–0.4 × distance from eye to nearest lateral ocellus.
Mesosoma. Posterior-ventral margin of lateral pronotum weakly crenulate, nearly smooth for most of length. Precoxal sulcus parallel-sided, narrowly crenulate, short, weakly impressed anteriorly, not extending close to anterior margin of mesopleuron; precoxal sulcus at 45 degree angle, inclined more vertically than in Eurytenes abnormis. Notaulus distinctly impressed over anterior third of mesoscutal disc, crenulate over anterior 0.2–0.3; with moderately dense cluster of short setae at rugulose base of anterior declivity extending ventrally to cover much of anterior declivity; with widely spaced line of 3–4 longer setae extending posteriorly towards but not reaching cluster of scattered setae around midpit (Fig. 3). Propodeum with median carina extending over anterior 0.3 before bifurcating to form five-sided areola over posterior 0.7. Surface smooth to weakly rugose laterally and posteriorly, carinae forming areola not obscured by sculpture, entirely visible, areola varying from smooth to weakly rugose (Figs 34, 35).
Wings.Fore wing r-m at most pigmented at extreme base, largely tubular (with lateral boundaries distinct) over anterior half; m-cu distinctly postfurcal; 3M distinctly pigmented in basal third, gradually weakening and becoming depigmented distally. Hind wing m-cu indistinct.
Legs. Hind tibia 7.5–8.7 (m=8.1) × longer than maximum width.
Metasoma. Petiole 1.6–1.8 (m=1.7) × longer than apical width. Female ovipositor short but distinctly protruding, about 0.9 × length of mesosoma. Ovipositor about 0.5 × length of mesosoma.
Color. Head and mesosoma dark reddish-brown as in Eurytenes abnormis, but with pale spot adjacent eye similar to though weaker than the spot in Eurytenes dichromus. Scape and pedical butterscotch, flagellomeres medium brown; clypeus butterscotch with slight infuscation dorsally. Palps, mandible, tegula, petiole, and ovipositor as in Eurytenes dichromus. Metasoma posteriad petiole patterned as in Eurytenes dichromus but T2+3 paler, whitish medially and T2 more lightly infuscate laterally. Legs about as in Eurytenes dichromus, with hind legs often a little paler. Ovipositor sheath dark red-brown. Wings hyaline.
Same as female except length of body 1.97–2.05 mm (m=2.01), length of fore wing 2.0–2.4 mm (m=2.3). Antenna with 24–28 flagellomeres; first flagellomere length 3.0–3.5 × width (m=3.4), fifteenth flagellomere length 2.4–3.0 × width (m=2.6), fifth from last flagellomere length 2.0–2.5 × width (m=2.4). Clypeus 1.3–2.3 (m=1.8) × wider than distance between clypeus and eye. Hind tibia 7.5–8.3 (m=7.9) × longer than maximum width. Petiole 2.0 × longer than apical width. Metasoma butterscotch dorsally with medium brown lateral banding on T2 and T3.
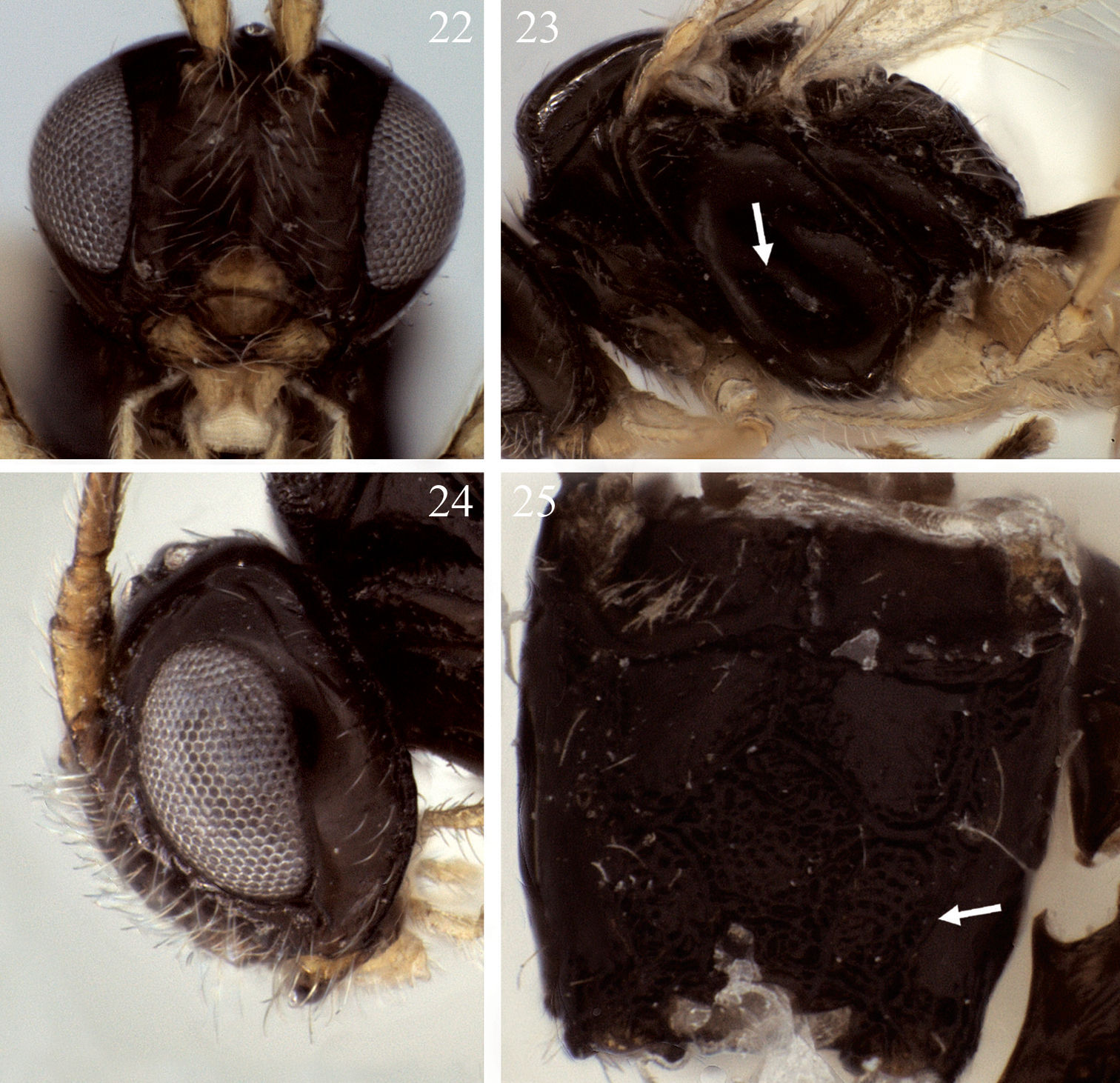
Eurytenes ormenus sp. n.22 Face 23 Mesosoma, lateral view, arrow = precoxal sulcus 24 Head, lateral view 25 Propodeum, dorsal view, arrow = sculptured posterior-lateral field.
Unknown.
Central Texas.
The name microsomus is derived from Greek: micro, small; somus, body. The name refers to the smaller size of this species compared to other species of Eurytenes.
Eurytenes microsomus and Eurytenes dichromus both occur in Austin, the westernmost locality for either species.
urn:lsid:zoobank.org:act:1CFFE835-69F9-49B3-8CE8-2FBDD05D5918
Fig. 5, 22–25♀ (TAMU): MEXICO: Guerrero 6.4 mi SW Filo de Caballo 9000 ft VII-8–1987 R. Wharton [five lines on a single label].
(TAMU): 1 ♀, same data as holotype except collected by Woolley and Zolnerowich; 1 ♀, Guerrero, 7 mi SW Filo de Caballo, 12.vii.1985, J. Woolley and G. Zolnerowich.
This species is most readily recognized by the dark brown hind femur. All other species from the New World have relatively pale (whitish to dark yellow) hind femora. The petiole is completely dark, as in Eurytenes pachycephalus, sp. n., but the latter is a much larger species with a distinctly broader gena.
(♀). Length of body: 1.9–2.2 mm (m=2.0), length of fore wing 2.8–3.2 mm (m=2.8).
Head. 27–29 flagellomeres; first flagellomere length 3.0–5.5 × width (m=4.3), fifteenth flagellomere length 3.5–4.0 × width (m=3.7), fifth from last flagellomere 3.5 × width. Face 1.4–1.6 (m=1.5) × wider than high. Clypeus nearly semi-circular in shape, with ventral margin truncate to very slightly concave (Fig. 22); 1.7–2.1 (m=1.95) × wider than high; 1.2–1.5 (m=1.35) × wider than distance between clypeus and eye. Mandible not obviously expanded basally. Gena relatively narrow (Fig. 24). Occipital carina extending about 0.7–0.8 × distance from eye to nearest lateral ocellus.
Mesosoma. Posterior-ventral margin of lateral pronotum distinctly impressed, varying from crenulate to nearly smooth for most of length. Precoxal sulcus weakly impressed, not extending close to anterior margin of mesopleuron; precoxal sulcus approximately 30 degrees, inclined slightly less vertically than Eurytenes abnormis. Notaulus narrow, weakly impressed, crenulate over anterior 0.3 of mesoscutal disc; with relatively sparse cluster of short setae at finely rugulose base of anterior declivity and 1–2 widely spaced longer setae extending posteriorly. Propodeum with median carina present anteriorly, bifurcating near anterior 0.2 to form five-sided areola over posterior 0.8; surface densely punctate-rugose to coarsely granular laterally and posteriorly, obscuring carinae, weakly sculptured to nearly smooth anteriorly on either side of short median carina.
Wings. Fore wing r-m very weakly pigmented at extreme base; somewhat tubular (with lateral boundaries distinct) over anterior 0.3–0.5; m-cu distinctly postfurcal; 3M distinctly pigmented in basal third, gradually weakening and becoming depigmented distally. Hind wing m-cu indistinct.
Legs. Hind tibia 8.7–9.5 (m=9.1) × longer than maximum width.
Metasoma. Petiole 2.3–2.9 (M=2.65) × longer than apical width. Female ovipositor short but distinctly protruding, about 0.9 × length of mesosoma. Ovipositor sheath about 0.5 × length of mesosoma.
Color. Head, thorax, and petiole dark red-brown. Scape and pedicel yellow, first four flagellomeres light brown, quickly darkening distally to dark brown; palps, mandible, clypeus, and tegula yellow. Metasoma posteriad petiole medium brown. Legs yellow except hind femur medium to dark brown medially with apical and basal 0.1–0.15 pale, tibia and tarsus almost completely medium brown, tibia variously pale brown dorsally. Ovipositor sheath dark brown, ovipositor light brown. Wings hyaline.
Unknown.
South central Mexico.
The name ormenus is derived from Greek: ormenus, petiolated. The name refers to the elongate petiole of the species.
This is a small-bodied species similar in size to Eurytenes microsomus but with a more heavily sculptured propodeum and darker hind femur. Eurytenes ormenus is characterized by the long, narrow petiole, similar in form to the petiole of Eurytenes pachycephalus sp. n. and Eurytenes abnormis and unlike the broader petiole of Eurytenes microsomus and Eurytenes dichromus. As in Eurytenes pachycephalus sp. n., and unlike the other three species, the petiole is uniformly very dark in coloration. The anterior tentorial pits of Eurytenes ormenus are slightly larger in this species than in the others treated here.
urn:lsid:zoobank.org:act:422562A0-496F-44C1-B403-B206893B2DB3
Fig. 6, 26–29♀ (TAMU): Mexico: Michoacan 6 mi. N. Cheran 8-VII-1985 Woolley & Zolnerowich [four lines on a single label].
(TAMU): 10 ♂, same data as holotype except eight with date 7–8.vii.1985.
This species is most readily recognized by its broad clypeus and inflated gena. It is a much larger species than Eurytenes ormenus, which was also collected at high elevation sites in central Mexico. Although both Eurytenes pachycephalus and Eurytenes ormenus have a uniformly dark petiole, the hind femur is dark in Eurytenes ormenus and more lightly colored in Eurytenes pachycephalus.
(♀). Length of body: 2.9 mm, length of fore wing 3.3 mm.
Head.36 flagellomeres; first, fifteenth, and fifth from last flagellomere length 3.3, 2.3, 2.3 × width, respectively. Face 2.0 × wider than high. Clypeus broad, semi-elliptical, with ventral margin truncate, 2.9 × wider than high; 2.1 × wider than distance between clypeus and eye. Mandible gradually expanded basally, without distinct basal tooth or swelling. Gena broad (Fig. 28). Occipital carina extending about 0.5 × distance from eye to nearest lateral ocellus.
Mesosoma. Posterior-ventral margin of lateral pronotum strigose for most of length, the sculpture extending towards middle of sclerite. Precoxal sulcus extending very close to anterior margin of mesopleuron; deeply crenulate anteriorly, sculptured area broadening posteriorly; precoxal sulcus approximately 45 degrees, inclined slightly more vertically than Eurytenes abnormis. Notaulus distinctly impressed and crenulate over anterior 0.3–0.4 of mesoscutal disc; with cluster of short setae at rugulose base of anterior declivity extending ventrally to some extent onto anterior declivity at each side; longer setae absent posteriorly. Median carina extending over anterior 0.2 before bifurcating to form five-sided areola; surface of areola and lateral margin of propodeum rugose, posterior-lateral fields and region anteriorad areola smooth or nearly so.
Wings. Fore wing r-m very weakly pigmented at extreme base, largely spectral (with lateral boundaries indistinct); m-cu distinctly postfurcal; 3M distinctly pigmented and largely tubular in basal third, gradually weakening distally. Hind wing m-cu extending nearly half way to wing margin as a very weakly pigmented and impressed curved line.
Legs. Hind tibia 8.3 × longer than maximum width.
Metasoma.Petiole 2.1 × longer than apical width. Female ovipositor sheath barely visible due to postmortem changes in position; visible portion densely setose.
Color. Head, mesosoma, and petiole black. Scape and pedicel yellow, flagellomeres dark brown; mandible butterscotch with distal tip infuscated; clypeus dark brown dorsally, ventral half butterscotch; palps and tegula butterscotch. Metasoma with T2 and T3 brown, middle tergites with brown and yellow transverse banding, apical tergites yellow. Legs yellow except hind tibia butterscotch to weakly infuscate, tarsus entirely medium brown. Wings largely hyaline, though appearing very slightly darker than other species treated here.
Male. Same as female except length of body 2.9–3.1 mm (m=3.0), length of fore wing 3.1–3.7 (m=3.4). Antenna with 34–38 flagellomeres; first flagellomere length 2.3–3.0 × width (m=2.8), fifteenth flagellomere length 2.0–2.35 × width (m=2.25), fifth from last flagellomere length 1.8–2.2 × width (m=2.1). Face 1.9–2.1 (m=2) × wider than high. Clypeus 1.7–3.0 (m=2.3) × wider than distance between clypeus and eye. Precoxal sulcus shape variable, generally narrowing anteriorly and widening ventral-posteriorly. 1–3 longer, somewhat decumbent setae extending posteriorly from notaulus on mesoscutal disc. Fore wing r-m mostly unpigmented, usually tubular throughout (with lateral boundaries distinct). Hind wing m-cu usually extending more than half way to wing margin as a weakly pigmented, spectral impression. Hind tibia 7.4–8.5 (m=7.9) × longer than maximum width. Petiole 2.1–2.7 (m=2.35) × longer than apical width.
Head and petiole dark reddish-brown to black; hind tibia usually more distinctly brown; tergites butterscotch with transverse bands of medium brown on T2-T4 and uniformly medium brown on T5 and following.
Eurytenes pachycephalus sp. n. 26 Face 27 Mesosoma, lateral view, arrow = precoxal sulcus 28 Head, lateral view 29 Petiole, dorsal view.
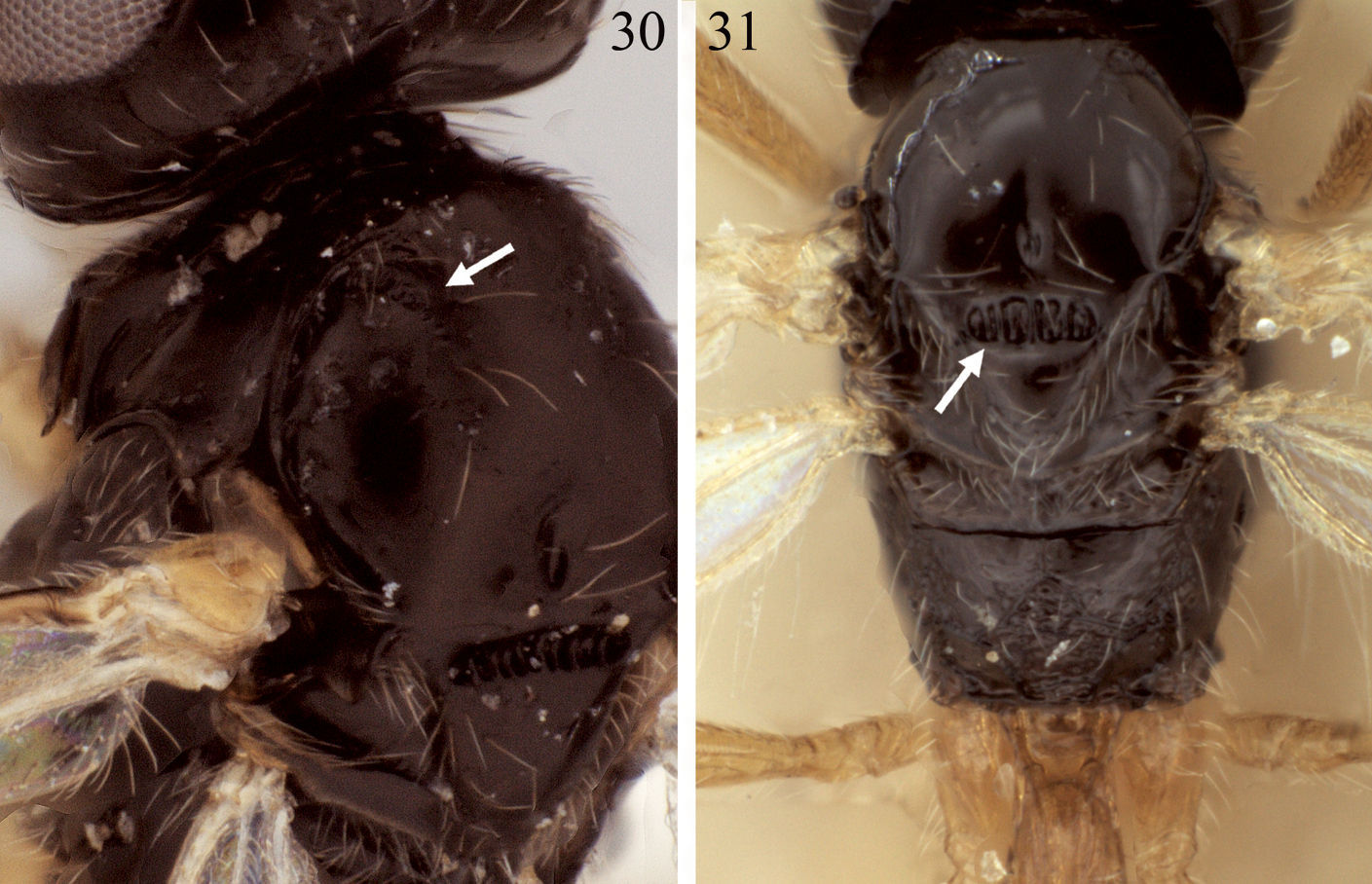
30 Eurytenes dichromus sp. n., mesoscutum, dorsal-lateral oblique, arrow = notaulus 31 Eurytenes abnormis, mesosoma, dorsal view, arrow = scutellar sulcus.
Propodeal sculpture patterns. 32–33 Eurytenes dichromus sp. n. 34–35 Eurytenes microsomus sp. n.
The name pachycephalus is derived from Greek: pachy, fat; cephalus, head. The name refers to the larger size of the head, extended gena and broad clypeus of this species.
Unknown.
Central Mexico.
We have selected the lone female as the holotype, for ease in comparison with the other species described here, even though the female specimen is not in the best of condition. The hind legs of this species are shorter and broader than those of Eurytenes ormenus.
We are most grateful to the following curators or collection managers and institutions for providing access to material used in this study: David Wahl (AEI), Paul Dessart (deceased, Institut Royal des Sciences Naturelles de Belgique), Bill Mason (deceased, Canadian National Collection), Jeno Papp (Natural Museum of Natural History, Budapest), Max Fischer and Herbert Zettel (NHMW), Paul Marsh and Bob Kula (USNM), and John Sivinski (USDA/ARS, Gainesville, Florida). We also thank Matt Yoder (NC State University) for his support with mx and two anonymous reviewers for useful suggestions. This work was funded by the National Science Foundation Partnerships for the Enhancement and Education in Taxonomy (NSF-PEET) Grant DEB 0328922 and associated REU supplement 1026618.