(C) 2010 Natalia A. Matushkina. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The sting microsculpture of the digger wasp Bembix rostrata (Fabricius, 1781) (Hymenoptera, Crabronidae) is studied with the scanning electron microscope (SEM) for the first time. As in many other hymenopterans, the second valvifer of B. rostrata possesses two fields of styloconic sensilla (hair plates) of proprioceptive function. The presence of two paired fields of campaniform sensilla on the second valvula and second valvifer is first shown in an apoid wasp. The first and the second valvulae bear scattered sensilla-like structures on the external surface, more numerous apically. The first valvula has two subapical barbs externally and a pair of valvilli on its inner surface, whereas the outer surface of the second valvula is smooth. The third valvula is sclerotized externally, consisting of proximal and distal parts, and bearing four sensilla morphotypes of mechanoreceptive and probably chemoreceptive functions. The inner surface of the valvulae and the membranous cuticle that is touching the sting have microstructures of different shapes directed distally. Functional aspects of characters studied are discussed.
Morphology, sting apparatus, ovipositor
The Hymenoptera
are the sole group of endopterygote insects with a well-developed
ovipositor, a plesiomorphic retention that has been considered one of
the key factors in their diversification (
There are numerous classic works on morphology and functions of the hymenopterous ovipositor (e.g.
Six females of Bembix rostrata were collected in Central Ukraine (Kyiv Province, Vyshgorodsky District, surroundings of village Khotyanivka, July 2008; 50°34'55"N, 30°33'51"E). The gaster was removed from the wasp body using forceps, cut open slightly and macerated in 10% KOH. The sting apparatus was subsequently excised from the genital chamber, washed in water and examined in glycerine under a stereo microscope. For SEM study, the cuticular parts were washed in distilled water, dehydrated in a graded ethanol series and acetone, critical point dried (OM CPD 7501), coated with gold-palladium (OM-SC7640) and examined with a Zeiss EVO-50 SEM (Museum of Zoology, Natural History Senckenberg Collections Dresden, Germany).
The terms used are preferably from
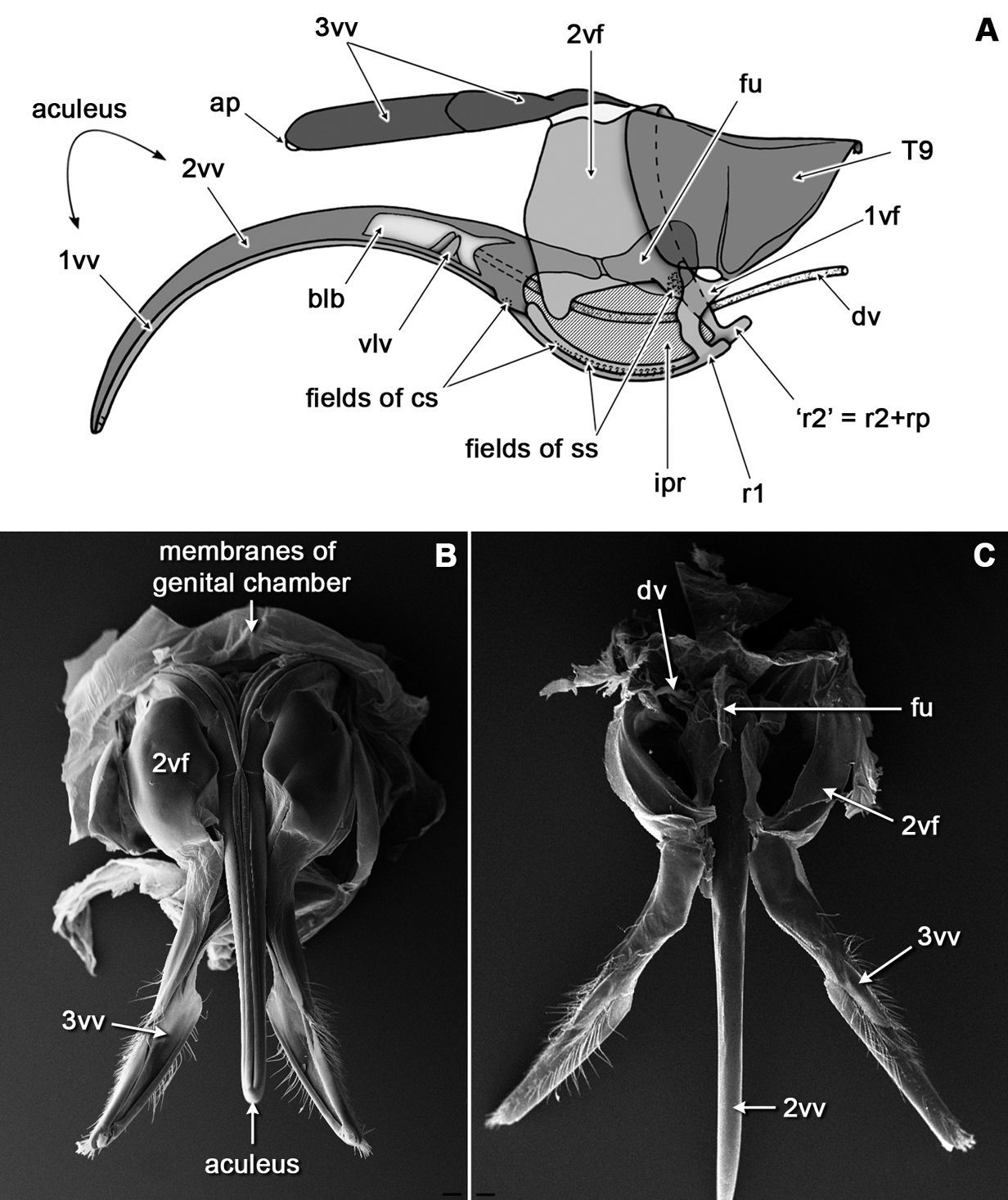
(Figs 1, 2A, B). The sting apparatus of Bembix rostrata lies within the genital chamber formed by the partial infolding of the 8th and 9th abdominal segments (7th and 8th metasomal segments) into the 7th abdominal segment, thus only the apical part of the sting is visible outside at rest. The sting shaft itself (= aculeus) is massive, curved ventrally and comprises paired 1st valvulae (= gonapophyses of 8th segment, ventral valves and lancets) and unpaired 2nd valvulae (= medially fused gonapophyses of 9th segment, dorsal valve, and stylet). Basal parts of the 1st and 2nd valvulae are continuous with long curved dorsally processes (rami) which extend to the valvifers: the 1st ramus is connected to the 1st valvifer (= gonangulum; triangular plate), whereas the 2nd ramus is fused laterally with the anterior region of the 2nd valvifer, the region called the rostral process. The 1st and 2nd valvulae (and their rami) form a sliding interlocking mechanism called the olistheter which comprises a groove-like ventral component (= aulax) and a tongue-like dorsal one (= rhachis). The unpaired furcula attaches to the base of the 2nd valvula highly flexibly. The 1st valvifer possesses two articulations: the anterior one is with the modified 9th tergite (= quadrate plate), the posterior one with the 2nd valvifer (= proximal part of 9th gonocoxite). The very elastic membraneous incision (= incisura postarticularis, postincision) separates the anterior rostral process of the 2nd valvifer from its main body. The sting itself is ensheathed by paired 3rd valvulae (= distal part of 9th gonocoxite or sting sheaths) when not in use. The modified 8th tergite (= spiracle plate) is the outermost part of the sting apparatus and is connected neither with the sting valves nor with the valvifers.
General organization of the sting apparatus in the digger wasp Bembix rostrata: A diagram showing relative position of main parts in a lateral view (aculeus is extended ventrally, furcula is turned anteriorly; bulb is shown in longitudinal section to show valvilli, the membranous incisura postarticularis is hatched); B-C, SEM micrograph of the sting in ventral B and dorsal C views. Scale bars: 100 μm.
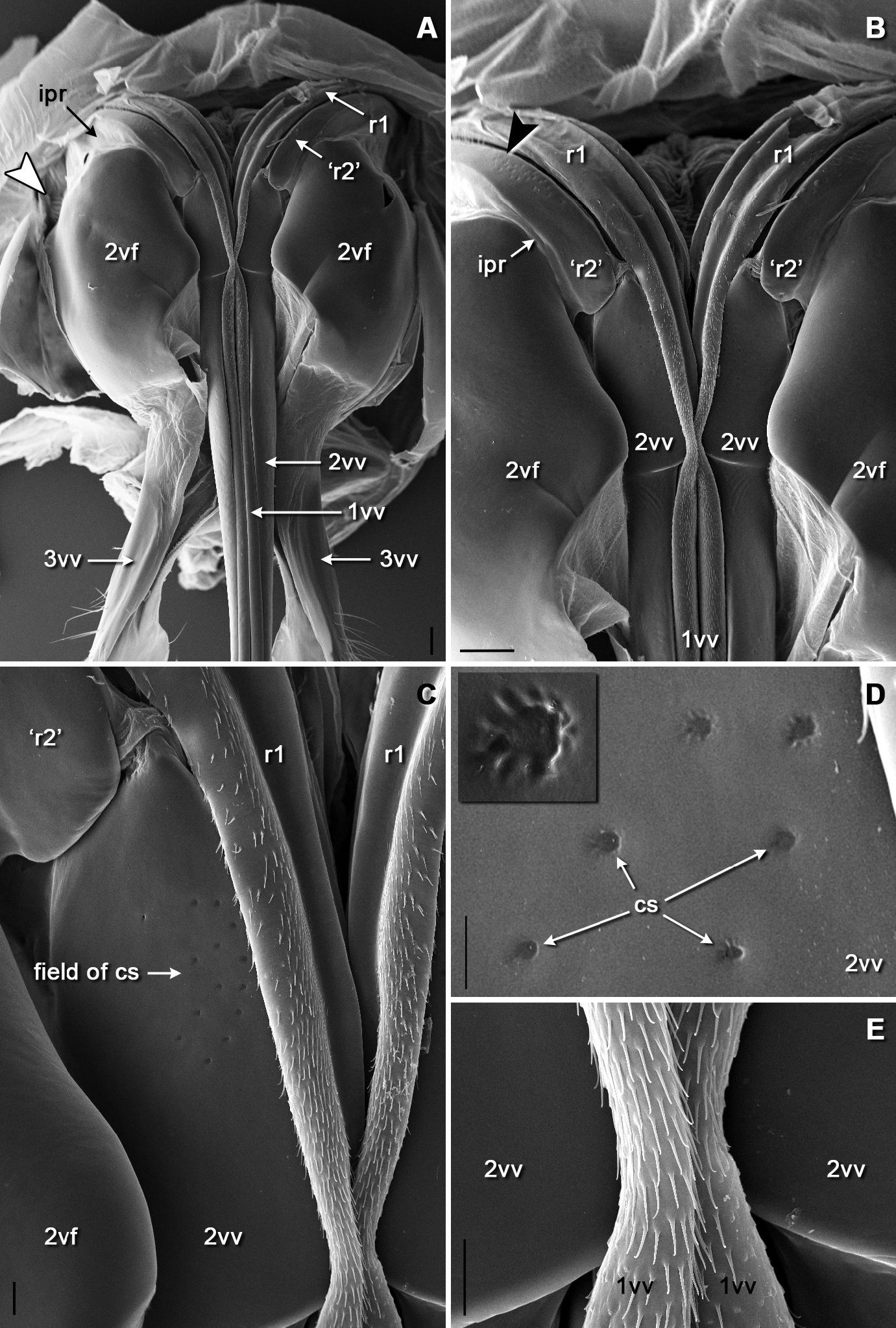
SEM micrographs of the sting basis, ventral view: A general aspects B basal region of the rami of both valves and of the second valvifera C field of campaniform sensilla on basis of the second valve D detail of the field of campaniform sensilla on basis of the second valve (one campaniform sensillum enlarged in inset) E distally directed microspination on membraneous regions of the first valves. Arrowhead in A shows position of the field of styloconic sensilla on the second valvifer nearby its articulation with the first valvifer (enlarged in Fig. 3B). Scale bars: 100 μm in A and B; 20 μm in C and E; 10 μm in D.
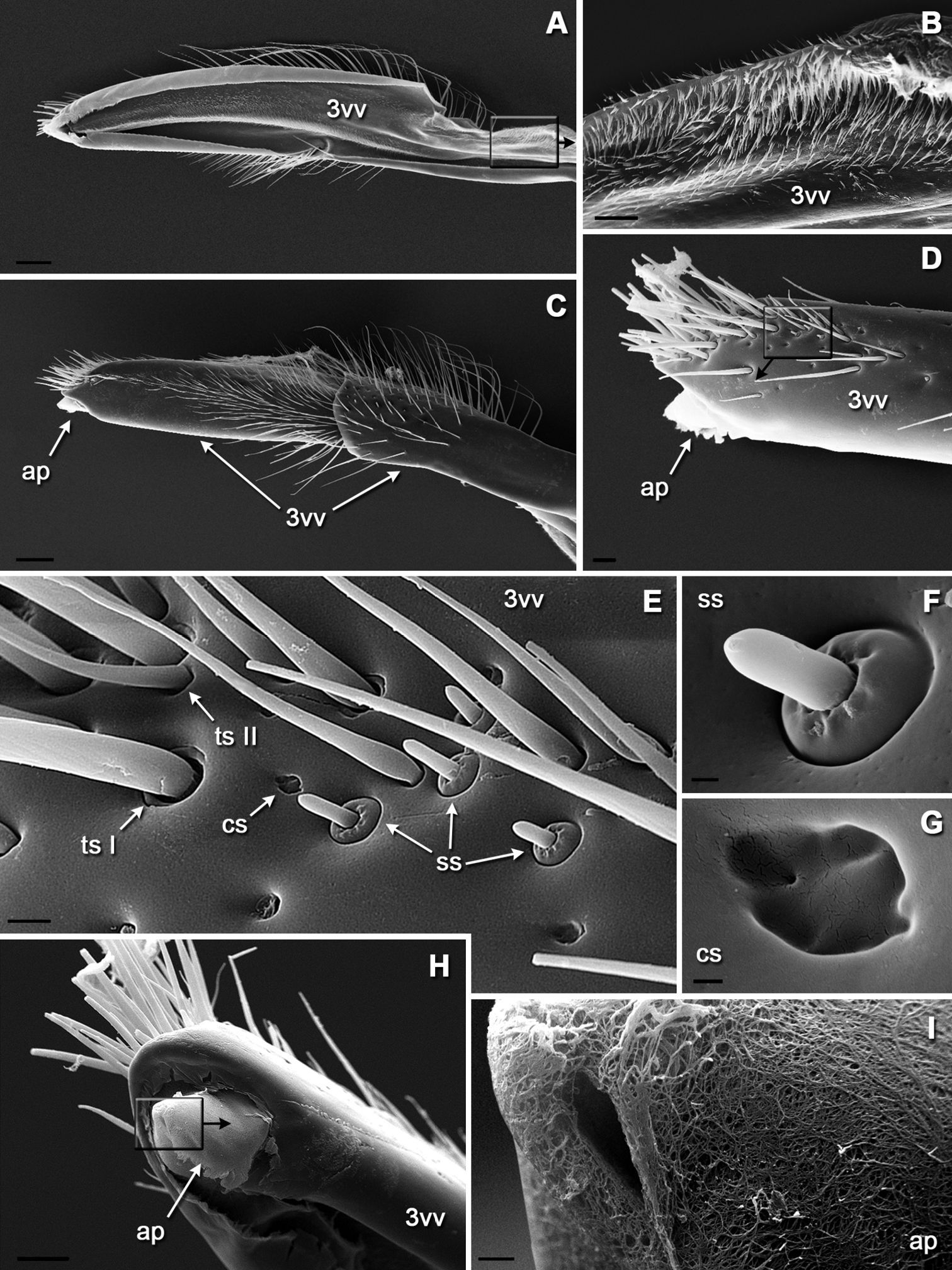
SEM micrographs of the sensilla on the second valvifer: A position of two fields of sensilla on the articular process of second valvifer, ventral view B field of styloconic sensilla nearby the articulation with first valvifer, lateral view C detail of the row of styloconic sensilla on the articular process of the second valvifer D detail of the field of campaniform sensilla on the articular process of the second valvifer distally to the row of styloconic sensilla. Insets in A are enlarged in C and D. Scale bars: 30 μm in A; 10 μm in B and C; 2 μm in D.
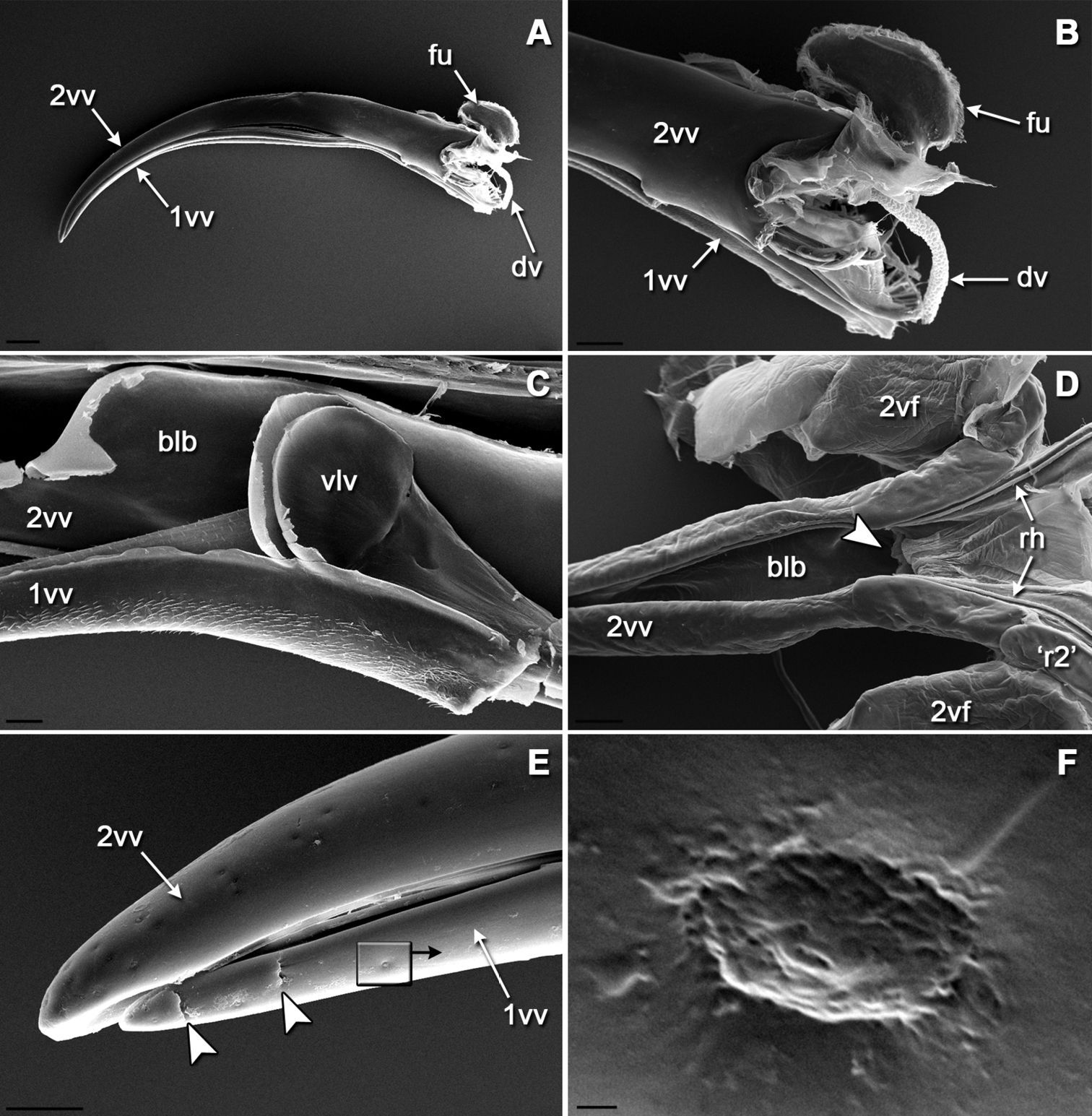
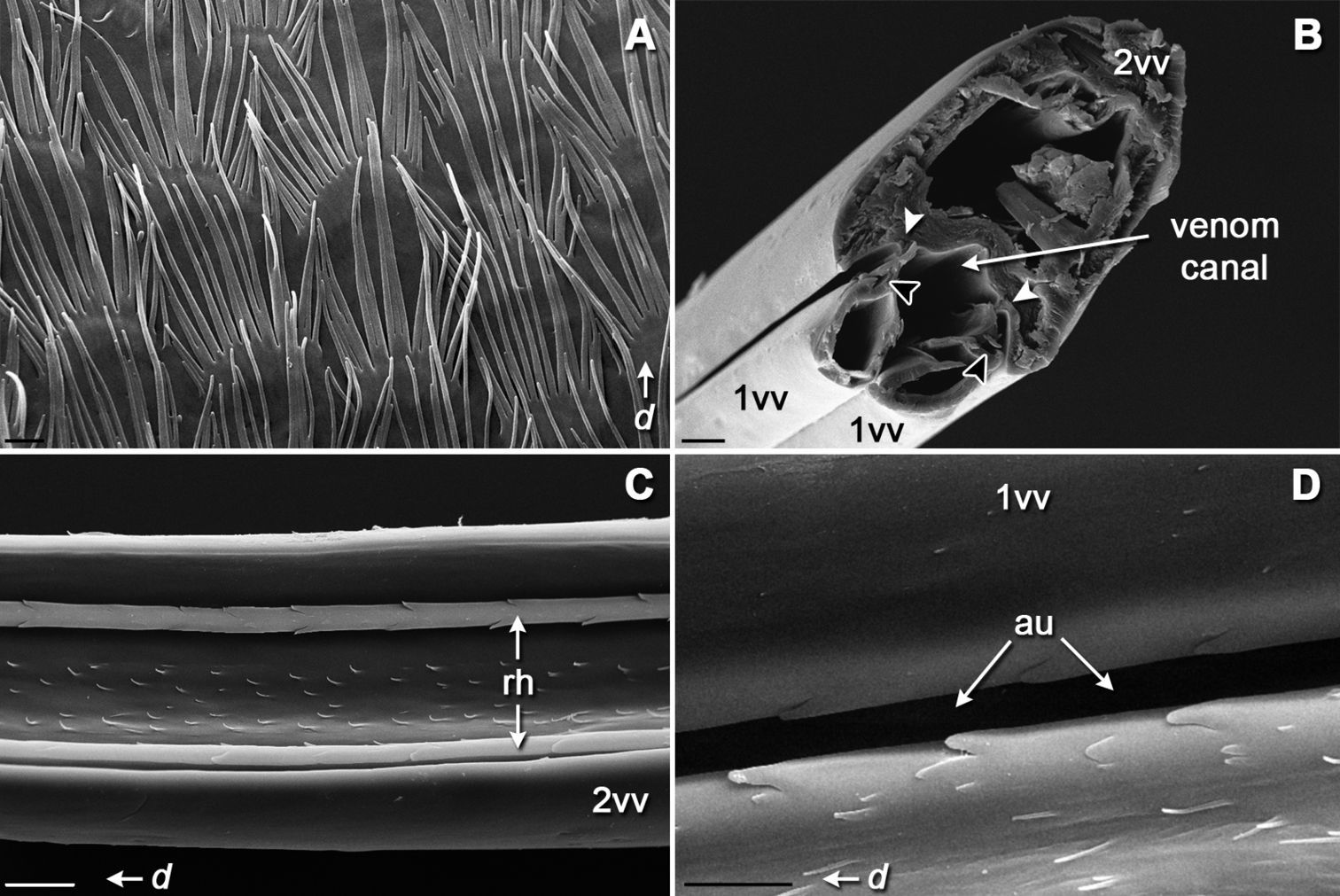
The entire external surface of the sting proper is mostly smooth, with only two subapical barbs on the 1st valvula (Figs 4A, E); however, there are numerous sensilla-like structures scattered over the entire surface, these are more numerous apically (Figs 4E, F). The dorsal surface of the 1st valvula forms an anterior swelling where two flaps called valvilli arise (Fig. 4C). These valvilli are housed within a broadened part of the 2nd valvula called the bulb. The venom duct enters the sting base at the anterior margin of the bulb (Figs 4A, B, D). Components of the olistheter are finely serrated, and the inner surface of the 2nd valvula bears distally directed microtrichia (Figs 5B–D). The setose membrane of the genital chamber covers the sting base (Fig. 5A). Three fields of sensilla are found on the 2nd valvifer. A row of styloconic sensilla is situated on the fused 2nd ramus and rostral process (Figs 3A, C). The seta of each sensillum in a row may touch the 1st ramus, and the more the 1st valvula protracts relative to 2nd valvula the more sensilla contact the 1st ramus. A field of 25–28 uniformly directed campaniform sensilla is situated more distally (Figs 3A, D). Another group of at least 35 styloconic sensilla that forms a setal plate is situated on the main sclerite of the 2nd valvifer where it articulates with the 1st valvifer (Figs 2A, 3B). The seta of each sensillum in this field may touch the 1st valvifer. A paired field of ca. 13 uniformly directed campaniform sensilla is also found on the anterolateral surface of the 2nd valvula where it articulates with the rostral process (Figs 2C, D). The well sclerotized 3rd valvula consists of two segments, the proximal and the distal (Figs 6A, C). Its inner wall is mainly membranous with a densely microsetose surface and bears a longitudinal ridge (Figs 6A, B). Apically, the inner membrane of the 3rd valvula forms a cone-shaped process composed of a peculiar spongy cuticle (Figs 6C, H, I). At rest, this apical process abuts closely to the dorsolateral surface of the 2nd valvula. There are at least four morphotypes of sensilla on the 3rd valvula (Figs 6D–G). Trichoid sensillum type I is socketed and has a rounded setal tip; these sensilla are aggregated apically. Trichoid sensillum type II is unsocketed and sharply pointed; these sensilla are the most abundant type, they are located all over the external surface but mostly at the apex and at the margin between proximal and distal parts of the 3rd valvula. Campaniform sensilla are rare and scattered among trichoid ones. Socketed styloconic sensilla bear a distinct pore on their rounded setal tip; these sensilla are found only on the dorsal surface of the 3rd valvulae between trichoid sensilla type I.
SEM morphology of the sting: A lateral view of sting with the furcula and venom duct B basal part of the sting C valvilli on the first valve positioned in the bulb of the second valve, medial view D ventral view of isolated second valve showing the bulb E lateral view of sting apex showing scattered sensilla-like structures on both valves and two subapical ridges on the first valve F single wrinkled sensilla-like structure that superficially resembles the secretory pores described by Nenon et al., 1995 (note characteristic contamination around the pore). Arrowheads indicate the position of the opening of the venom duct (in D) and the subapical barbs (in E). Scale bars: 200 μm in A; 100 μm in B and D; 30 μm in C and E; 300 nm in F.
SEM micrographs of internal surfaces of the sting apparatus: A setose membrane of the genital chamber that contact the sting basis B transverse section of the aculeus showing position of the parts of the olistheter C ventral view of isolated second valve showing internal microsculpture between two rhachises D dorsal view of aulax of first valve. d, distal direction. Arrowheads in B indicate position of rhachises (white) and aulaxes (black) of the olistheter mechanism. Scale bars: 3 μm in A and D; 20 μm in B and C.
SEM morphology of the third valve: A medial view (inset enlarged in B) B microspination of membraneous inner wall C lateral view D apex in lateral view (inset enlarged in E) E variety of sensilla on dorsoapical surface F socketed styloconic sensillum with apical pore G campaniform sensillum H apex in mediodorsal view showing membranous process (inset enlarged in I) I spongiform cuticle of an apical process. Scale bars: 100 μm in A and C; 20 μm in B and D; 5 μm in E; 1 μm in F; 0.5 μm in G; 30 μm in H; 2 μm in I.
Bembix rostrata
is one of the most widely distributed and remarkable digger wasps
species in Europe, and it often forms large colonies of dozen to
hundred individuals. It prefers sandy and sunny habitats, where a
female digs several burrows. As in most bembicine wasps, Bembix rostrata
paralyzes the prey, which are predominantly large tabanid and syrphid
flies, by inserting the sting through the venter of the thorax (
Several surfaces of the sting apparatus of Bembix rostrata are covered with unidirectional microstructures. The wall of the venom canal in Bembix rostrata
is furnished with small microtrichia that are orientated distad. They
are randomly scattered and relatively sparse, and seem to be somewhat
reduced if compared with other non-aculeate hymenopterans where they
form a comb-like or ctenidial pattern (
The sensory equipment of the sting apparatus of Bembix rostrata
is diverse. The sensilla can be divided into those that perceive
intrinsic stimuli from the insect body (proprioceptors) and those
detecting environmental factors (exteroceptors) (
I am grateful to Dr. Yuri Protsenko (Kyiv National University, Ukraine) for identification of wasps, and to Prof. Neveen Samy Gadallah (Cairo University, Egypt) for her help with the literature. Dr. Wojciech J. Pulawski (California Academy of Sciences, San Francisco) is sincerely thanked for his moral support and his kind improvement of the draft version of the manuscript. I am also indebted to Prof. Laurence Packer (York University, Toronto, Canada) for careful reading of the manuscript and suggested corrections.