(C) 2011 Michael W. Gates. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
We describe and illustrate two techniques for enhancing curatorial and processing efficiency as it pertains to parasitic Hymenoptera (Chalcidoidea, Cynipoidea). These techniques were developed in response not only to the massive number of parasitoids that have been acquired through various biodiversity studies, but also the difficulty in mobilizing the human resources to curate this material. The first technique uses small, crystal polystyrene boxes with tight-fitting lids to store dehydrated specimens prior to mounting. Locality information is affixed to the box and specimens are spread in a layer for ease of examination by researchers. Solutions for managing static electricity within the specimen boxes are discussed. The second involves a vacuum pump connected to a funnel with a filtration membrane and flask apparatus to rapidly dehydrate hard-bodied parasitoids (Figitidae) that are not subject to collapse during air-drying.
Chalcidoidea, Cynipoidea, crystal polystyrene, vacuum pump, curation, dehydration
Participation in large-scale biodiversity studies and other ecological research projects (
After amassing a large volume of raw insect residues from biodiversity studies from which parasitic Hymenoptera must be separated, we employ an aqueous technique using mesh colanders, plastic tubs, and an orbital shaker to separate each raw residue into a macro and a micro fraction (
For many taxa (e.g. Chalcidoidea), hexamethyldisilazane (HMDS) is used as part of a chemical dehydration process required to prevent specimen collapse while air drying; we follow the protocols of
1 Miscellaneous vials for specimen storage 2 Ilford antistatic cloth. 3 Kinetronics antistatic brush. 4 Zerostat 3 product box. 5 Zerostat 3 gun with cap. 6 USNM ½ unit tray with plastic specimen boxes. 7 Specimens attracted to box lid by static. 8 Specimens at bottom of box lid after static dissipated.
Images of supplies, equipment and specimens were taken with either an Olympus PEN EP-1L (Figs 9–15) or a Canon Powershot A3100 digital cameras (Figs 1–8). The crystal polystyrene specimen storage boxes (rectangular cases) are manufactured by Caubère (http://www.caubere.fr/en/produits/carres_rectangulaires/carre_rectangulaire02.htm) and the size we use most frequently is the 56 × 41 × 6 mm. The antistatic cloth (13 × 13” Ilford Antistaticum), antistatic brush (Kinetronics Corp., Model SW-060), and antistatic gun (Zerostat, MiltyPro Zerostat 3) are inexpensive and available online (Table 1).
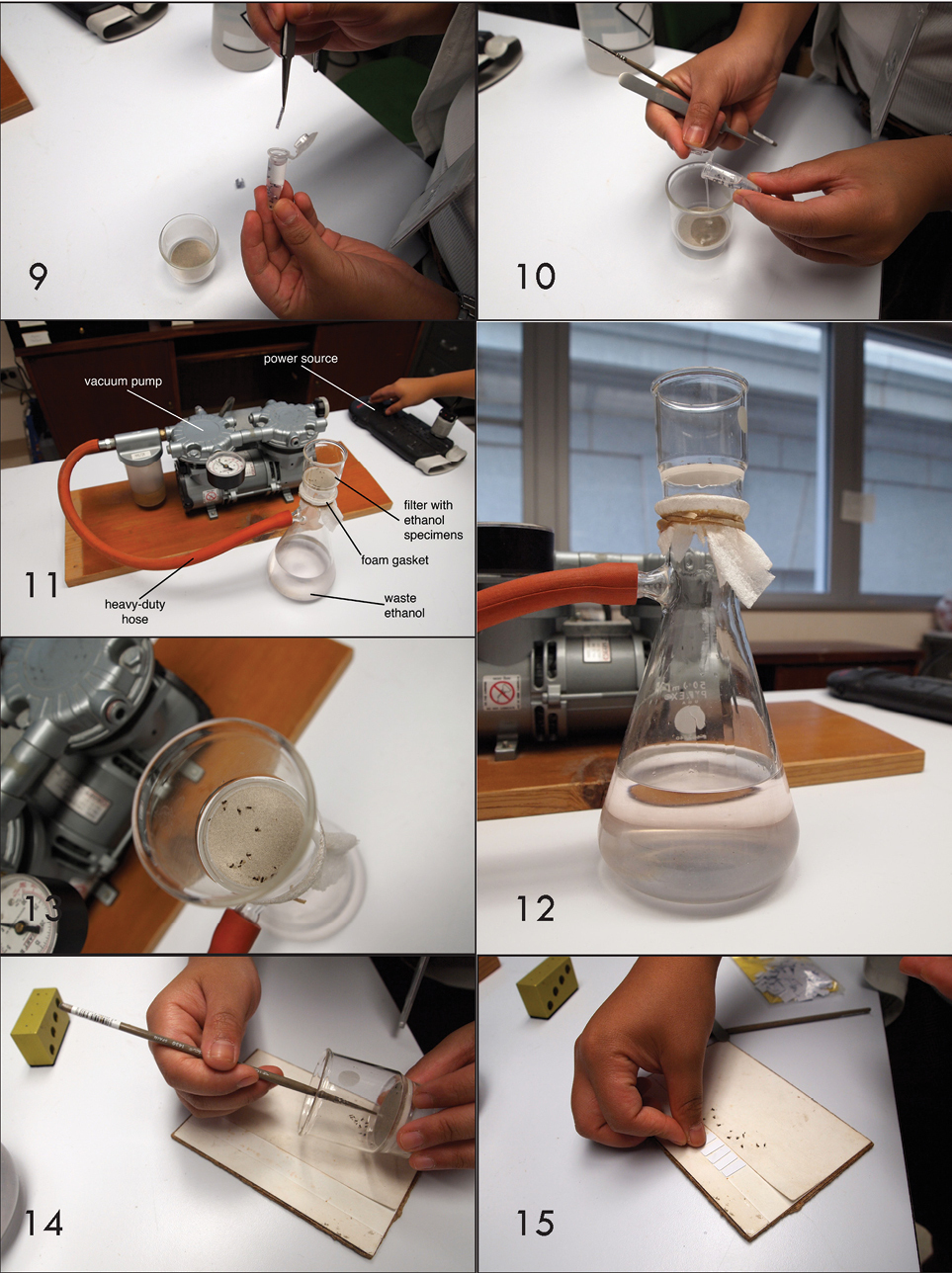
The vacuum pump is Gast LAA-V104-NQ, a model of oilless miniature rocking piston pump/vacuum; glassware associated with the pump are an Erlenmeyer flask, geological filter, styrofoam padding (for a gasket), and high-density hose. Another filter apparatus tested with success was a GE Disposable ‘All-in-one Vacuum Filtration Unit’ available from Fisher Scientific. The abbreviation for our collection is USNM (National Museum of Natural History, Smithsonian Institution, Washington, D.C., USA).
9 Collection labels are removed from the sample. 10 Specimens in ethanol are poured into the filter. 11 Complete vacuum pump system. 12 Filter under vacuum. 13 Specimens dried after running the pump. 14 Specimens removed from the filter onto a mounting board. 15 Glue boards are arranged.
Cost of supplies.
| Item | Cost | Source |
|---|---|---|
| Milty Zerostat 3 | $100 | http://www.buy.com/ |
| Ilford Antistatic Cloth | $8.00 | http://www.amazon.com |
| Polystyrene box (#546) | ~$100/500 | http://www.caubere.fr/en/produits/ (must contact for current pricing) |
| Kinetronics 60 Antistatic Brush | $19.95 | http://www.buy.com/ |
| FJC 6909 3.0 CFM Twin Port Vacuum Pump | $115 | http://www.amazon.com |
| Filtration kit w/ 70mm w/ buchner funnel, flask and filter paper | $18.95 | http://www.amazon.com |
Clear Box Specimen Storage. 1) Arrange workspace so that it is clean and lay down an antistatic cloth (Fig. 2) on which to work with specimens and boxes. 2) Brush the inner surfaces of the empty boxes (depending on local static electricity conditions, this may be unnecessary) with the antistatic brush (Fig. 3) to eliminate residual static. The blend of natural hairs and conductive synthetic fibers and conductive nature of the brush dissipate static effectively. 3) If static remains an issue, the Zerostat gun may be used (Figs 4, 5). We typically need to use it only on the inner surface of the lid in these conditions, especially if specimens “jump” onto the lid. To test, static was deliberately generated by rubbing the closed box with specimens on carpeting until specimens were attracted to the lid (Fig. 7). The box was opened with specimens on the lid (Fig. 7), the Zerostat was held approximately six inches away from the lid and activated. The specimens from the lid fell off as the static was dissipated. The gun contains two piezoelectric crystals and compression trigger which, by slowly squeezing the trigger, generates a stream of positive ions, and upon slowly releasing the trigger, releases a negative stream of ions. It requires no batteries and is durable (expect 10, 000 trigger pulls). 4) The locality label is affixed with clear tape underneath the bottom of the box (Fig. 7), the specimens are deposited into the box, and the lid is placed on the bottom. 5) The specimens are ready for storage in unit trays in drawers (Fig. 6). Based upon the box size used most frequently, 18 boxes easily fit in a standard USNM ½ unit tray for a total of 128 boxes per USNM drawer. Many sizes are available to fit different taxa.
Crystal polystyrene boxes possess several attributes that are favorable for dry storage: 1) transparency; 2) tight-fitting lids; and 3) high stiffness and dimensional stability. These attributes offer several advantages: 1) labels may be affixed to the bottom and can be viewed while simultaneously examining specimens under stereoscope; 2) enables rapid sorting of specimens not yet available as mounted and labeled material; 3) specimens may be removed for use in research projects as needed with minimal disturbance to non-target specimens; 4) specimens may be easily mailed to other researchers, avoiding disadvantages associated with shipping ethanol-preserved specimens (e.g. hazardous materials packaging, package preparation training, restrictions imposed by shipping companies).
Vacuum Dehydration. 1) Remove collection labels from the sample (Fig. 9). 2) Pour ethanol-preserved specimens into the filter (Fig. 10). For samples with an abundance of debris in the ethanol (e.g. lepidopteran scales), additional clean ethanol can be added to float off debris from the specimens. 3) The complete vacuum pump system is illustrated in Figure 11. In this example, the pump motor is always ‘on’, so the power strip also functions as the ‘on/off’ switch. Note the heavy-duty hose; this is critical because a weaker hose will collapse under vacuum. The foam gasket insures a firm seal between the filter and the flask below. 4) Filter under vacuum; ethanol is pulled through the membrane, specimens remain in filter (Fig. 12). Recycle or discard waste ethanol. 5) Dry specimens by running the pump for 30 seconds (Fig. 13). 6) Remove specimens from the filter onto a mounting board (Fig. 14). In cases where there are greater than 50 specimens, the majority can be removed by inverting the filter. Some specimens will remain on the filter membrane, and these can be removed using a micro paintbrush. 7) Arrange glue boards in preparation for affixing wasps (Fig. 15).
DiscussionResearchers attempting to examine dried, unsorted material stored in miscellaneous transparent or translucent vials face the challenge of searching for specimens of interest through the vial wall and within a specimen bolus of various sizes (e.g., Fig. 1). The alternative is the more time-consuming process of emptying the specimens on a tray and sorting through them under the stereoscope, before placing them back into the vial. Clear boxes solve both issues and allow the sorter to open a box, remove the specimens of interest, and mount them immediately for use or put them in a gelatin capsule. Advantages over long-term cold storage include no electrical requirements or storage issues related to flammable liquids. Previously, dried material was stored in a variety of vials of different sizes, making it difficult to efficiently sort specimens. If static is a problem, the Zerostat gun can be used for spot treatments.
Advantages for using the vacuum pump system for drying hard-bodied micro Hymenoptera include: 1) speed of curation dramatically increased (uninterrupted drying and mounting can achieve 500 specimens/day); 2) no need for hazadous chemical handling (including a fume hood, gloves, lab coat, and goggles); 3) low cost; once the vacuum pump system is assembled, there is no further investment required. Further, many laboratories are equipped with ‘lab-vac’ alongside compressed gas for burners. A lab equipped with such vacuum does not require the acquisition of a vacuum pump. In conjunction with the polystyrene boxes, thousands of specimens per day can be taken from ethanol and stored dried, awaiting examination at a later date.
Success using the vacuum pump drying technique can be influenced by the size of the arthropod being dried. In our experience, larger cynipoids that have thick cuticle (e.g. Liopteridae) tend to take longer to dry. In some instances, a specimen that appeared to look dry upon initial inspection clearly was not done drying when examined under a microscope while mounting. In these cases, ethanol could still be seen evaporating from setae. If this occurs, the specimens are returned to the vacuum apparatus and dried for a longer period of time.
Although the vacuum pump rapidly air-dries specimens, there exists the potentially deleterious side effect of damaging DNA due to the extended contact of specimen tissues with residual water (i.e. enzymatic cleavage in presence of water and oxidation) (
We gratefully acknowledge Gérard Delvare (CIRAD, Montpelier, France), who initially brought the crystal polystyrene boxes to our attention. We also thank Natalia Vandenberg and Thomas Henry (Systematic Entomology Laboratory [SEL], PSI, ARS, USDA) for their critical review of this paper. Jeffrey Sosa-Calvo (Smithsonian Institution, Department of Entomology) provided useful comments after reviewing this manuscript.