(C) 2011 Michael J. Sharkey. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The Thai fauna of eleven agathidine genera, i.e., Biroia, Braunsia, Camptothlipsis, Coccygidium, Cremnops, Cremnoptoides, Disophrys, Earinus, Gyrochus, Lytopylus, and Troticus, are revised. 25 species are treated, 20 of which are found in Thailand and five that are likely to occur there. Six new species are described, i.e., Braunsia chaweewanae Sharkey, sp. n., Camptothlipsis annemariae Sharkey, sp. n., Camptothlipsis sheilae Sharkey, sp. n., Coccygidium mastigion Sharkey, sp. n., Coccygidium phaeoscapos Sharkey, sp. n., and Cremnoptoides yui Sharkey, sp. n.The following new synonomies are proposed: Isopronotum seminigripenne Enderlein, 1920, Isopronotum tricolor Enderlein, 1920, Biroia soror van Achterberg & Long, 2010, are all synonymized with Biroia fuscicornis (Cameron, 1903). Braunsia pumatica van Achterberg & Long 2010 is synonymized with Braunsia fumipennis (Cameron, 1899). Braunsia devriesi van Achterberg & Long 2010is synonymized with Braunsia smithii (Dalla Torre, 1898). Cremnops malayensis Bhat, 1979 and Agathis nigritarsus Cameron 1899 are synonymized with Cremnops desertor (Linnaeus, 1758). Disophrys macilifera van Achterberg & Long 2010 is synonymized with Disophrys strigata Enderlein, 1920. Disophrys quymanhi van Achterberg & Long 2010 is synonymized with Disophrys subfaciata (Brullé, 1846). Agathis burmensis Bhat & Gupta (1977) is synonymized with Lytopylus ebulus (Nixon, 1950). Disophrys ornatipennis Cameron 1905 is transferred to Gyrochus ornatipennis Cameron, 1905, comb. n. Agathis flavipennis Brullé is transferred to Gyrochus flavipennis (Brullé, 1846), comb. n. A key to the genera of Thai Agathidinae and keys to the species of each genus with multiple species are presented.
Thailand, parasitoid wasp, Agathidinae, taxonomy, systematics
This is the first in a series of papers revising the agathidine braconids of Thailand. Here we treat species of the genera Biroia, Braunsia, Camptothlipsis, Coccygidium, Cremnops, Cremnoptoides, Disophrys, Earinus, Gyrochus, Lytopylus, and Troticus. The genera Aneurobracon, Bassus, Euagathis, Therophilus and Zelodia will be dealt with in subsequent publications. Twenty five species are treated here, twenty of which are found in Thailand and five of which have a high probability of being discovered in Thailand.
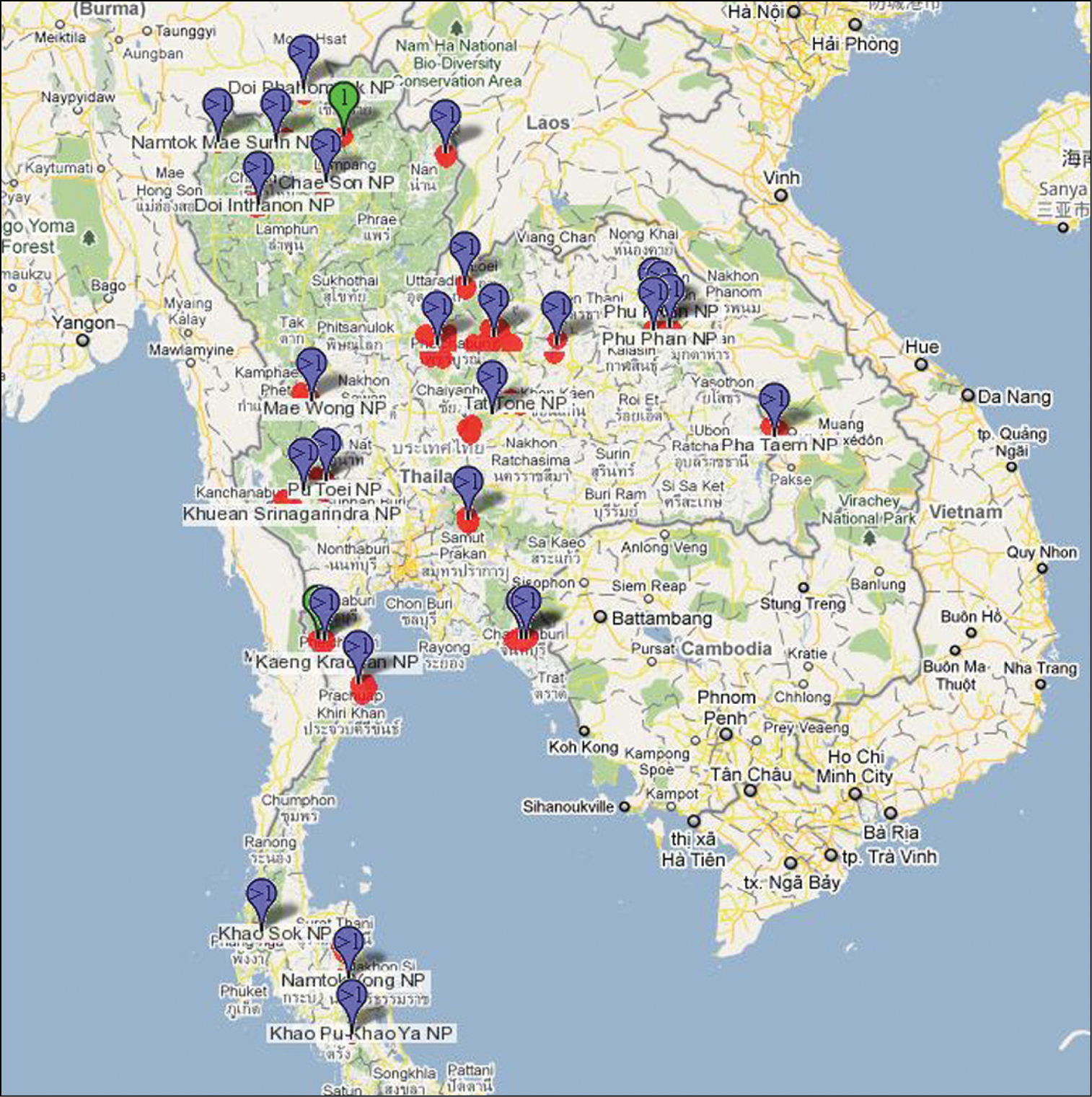
During the three year period 2006–2009, an intensive survey of the terrestrial arthropod fauna of Thailand was conducted through a collaborative effort among staff at Queen Sirikit Botanic Garden, The Thai Forestry Group, The Hymenoptera Institute and The Natural History Museum of Los Angeles County. This resulted in collecting at about 30 different parks and 559 different sites (Fig. 1). An electronic version of this map is available at the URL below figure 1. Malaise traps were the primary means of collecting, although pan traps and litter samples were also taken. The latter two methods did not result in any specimens of Agathidinae. Figure 1 shows the localities, mostly national parks, where intensive collecting was conducted with Malaise traps. The southern-most states of Thailand were not sampled due to political unrest in the area. For this reason we have included some agathidine species collected in Peninsular Malaysia, with the thought that they may occur in southern Thailand. Users of the species-level keys in this publication are encouraged to check the
Map showing collection sites in Thailand (Distribution map can be found on http://purl.org/thaimaps/all.
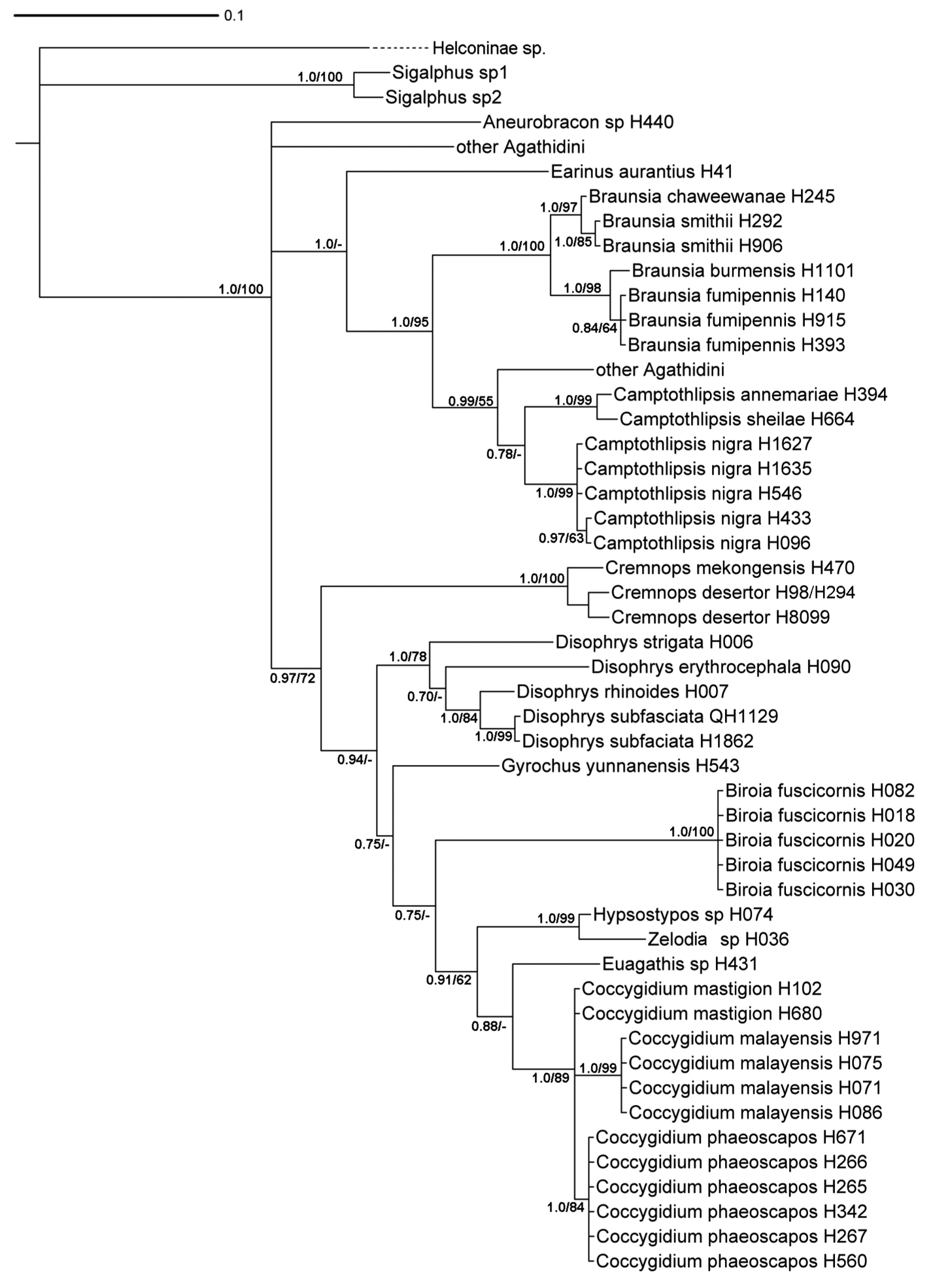
Species limits were ascertained using standard morphological characters and in several cases with the aid of 28S rDNA sequence data. We obtained 28S sequence data from a total of 41 specimens comprising 18 species. The reasons for the choice of specimens were several. 1. Some of the more variable species, based on morphology, were sampled to ensure that they were homogeneous for sequence data. For example, there was one particularly small specimen of what we thought to be Cremnops desertor. To check this hypothesis we compared the 28S data of this and a specimen of normal size. 2. Some of the species delimited on morphological grounds appeared to be separated on trivial morphological characters. For example the three species of Coccygidium treated here appeared to be different in general gestalt, but specific morphological differences were few and mostly based on color. Therefore this genus was rather heavily sampled, and our preliminary species definitions were corroborated, i.e., each of our morphological species was also distinguishable using 28S data, and every specimen of each species was identical. 3. In one case, Biroia fuscicornis, there are two rather distinct color forms, but otherwise no apparent morphological differences. Because the 28S data are identical between the two color morphs we decided that these represented color variation rather than separate species. 4. In one other case, Cremnops desertor, we tested the hypothesis that the Thai specimens were conspecific with those from Europe. Using a specimen from Sweden, the Thai and Swedish specimens differed in one base pair, thus corroborating our hypothesis. We used no magic numbers to determine species limits based on the 28S data. Some species showed some intraspecific variation, e.g., Camptothlipsis nigra (Fig. 2) whereas other species differed very little in 28S sequence data but were very distinct morphologically, e.g., Braunsia chaweewanae and Braunsia smithii (Fig. 2). The sequence data are summarized in a phylogenetic tree in figure 2.
Bayesian phylogram inferred from 28S (region D2-D3) sequence data aligned by secondary structure. Bayesian analysis runtime = 736, 000 generations (25% burnin). Branch support: Bayesian posterior probabilities / bootstrap (1, 000 replicates). (-) indicates bootstrap support below 60.
Regions D2-D3 of 28S rDNA (roughly 600 base pairs) were sequenced using the following primers: 28SD2hymF 5’ - AGAGAGAGTTCAAGAGTACGTG - 3’ and 28SD3hymR 5’ - TAGTTCACCATCTTTCGGGTC - 3’. Sequences were edited using Geneious Pro v4.7.5 (
Phylogenetic trees were constructed using maximum parsimony (MP) and Bayesian methods. MP was performed using TNT (
Source images for the dichotomous key and the interactive key are in Appendices 1 and 2. Keys were generated using DELTA Editor
Morphological terms follow
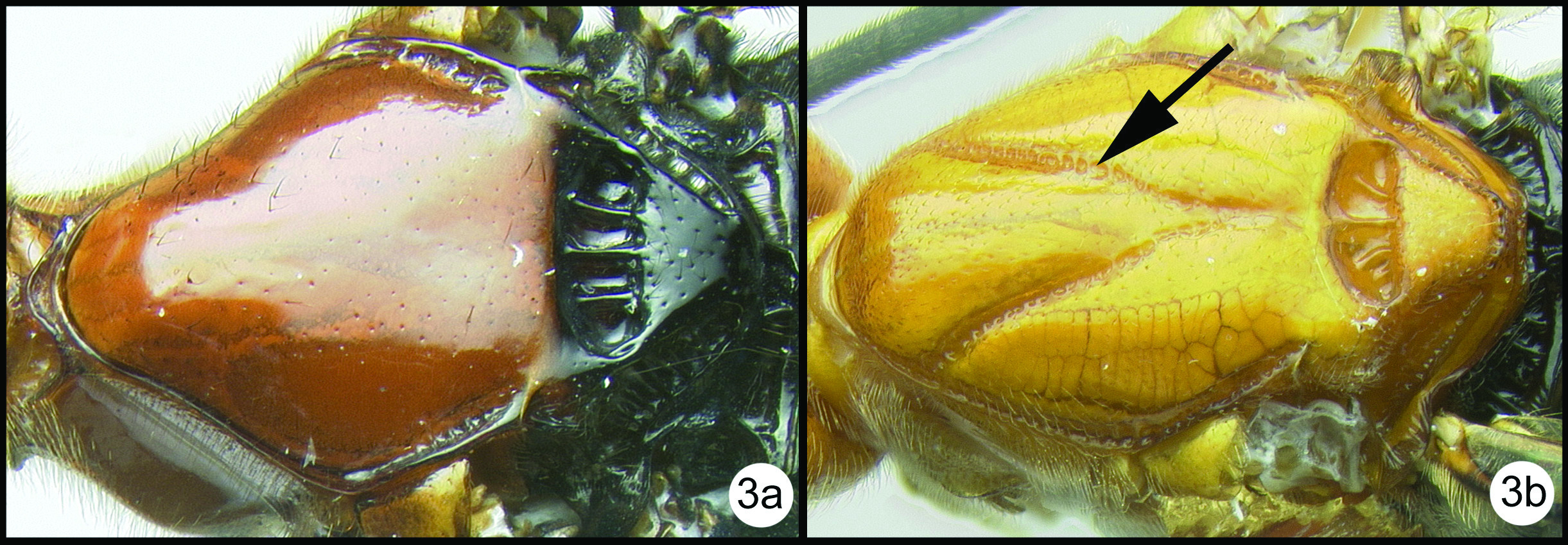
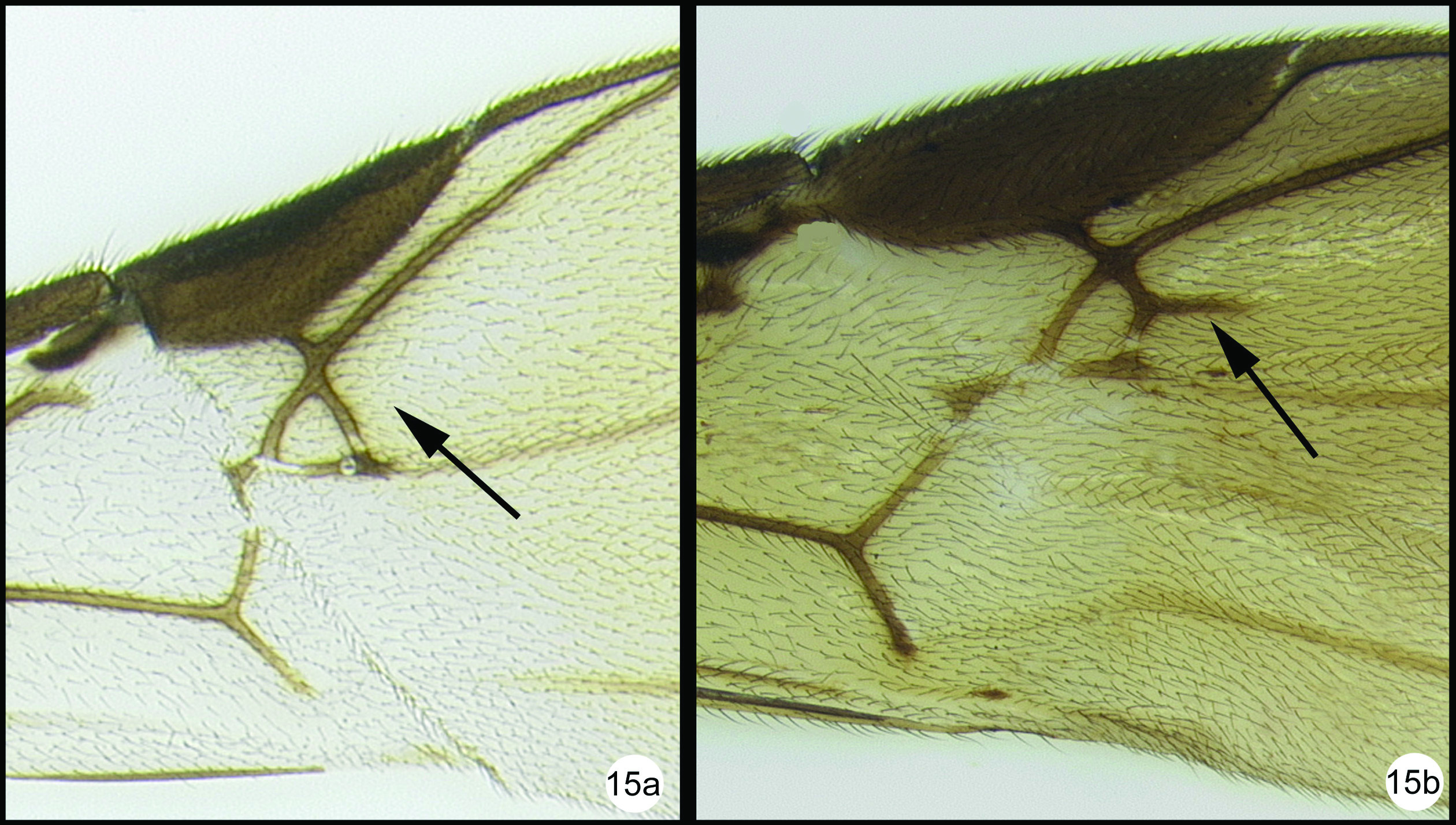
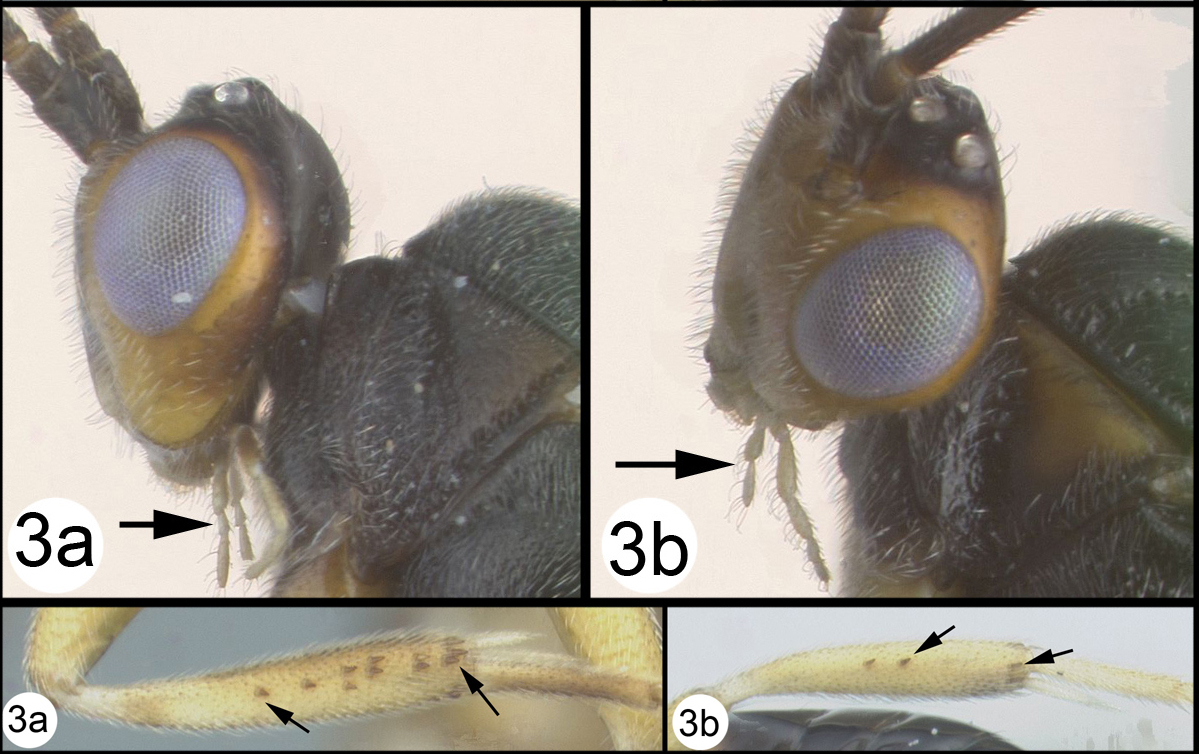
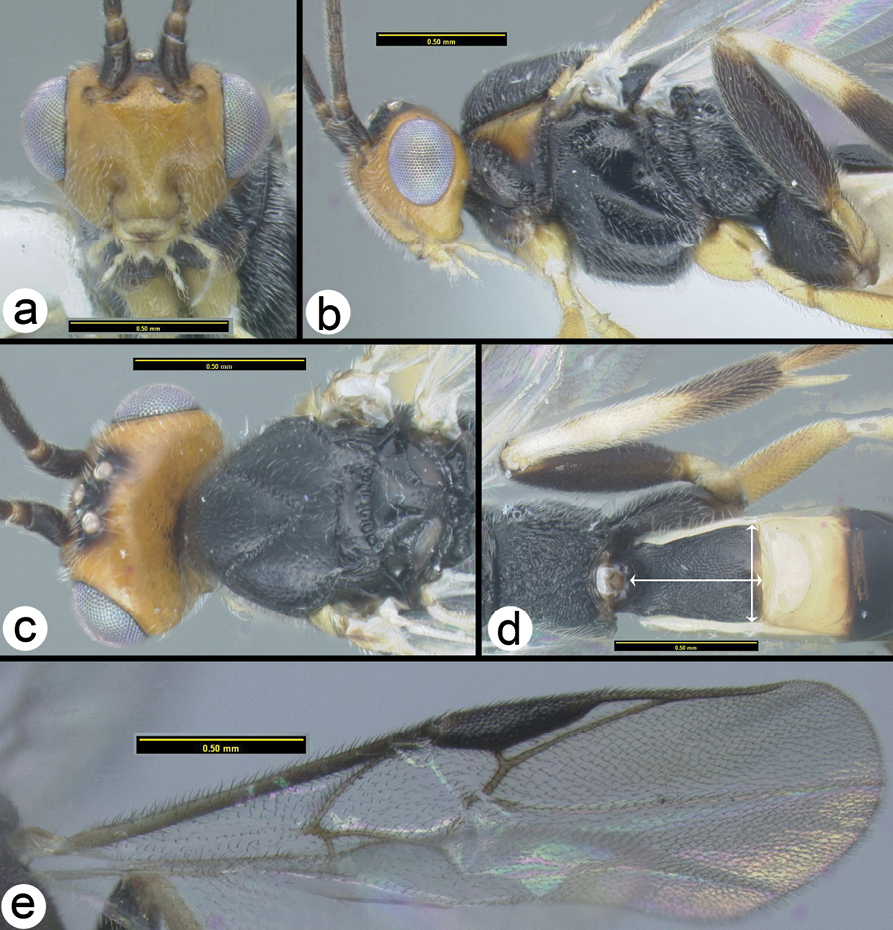
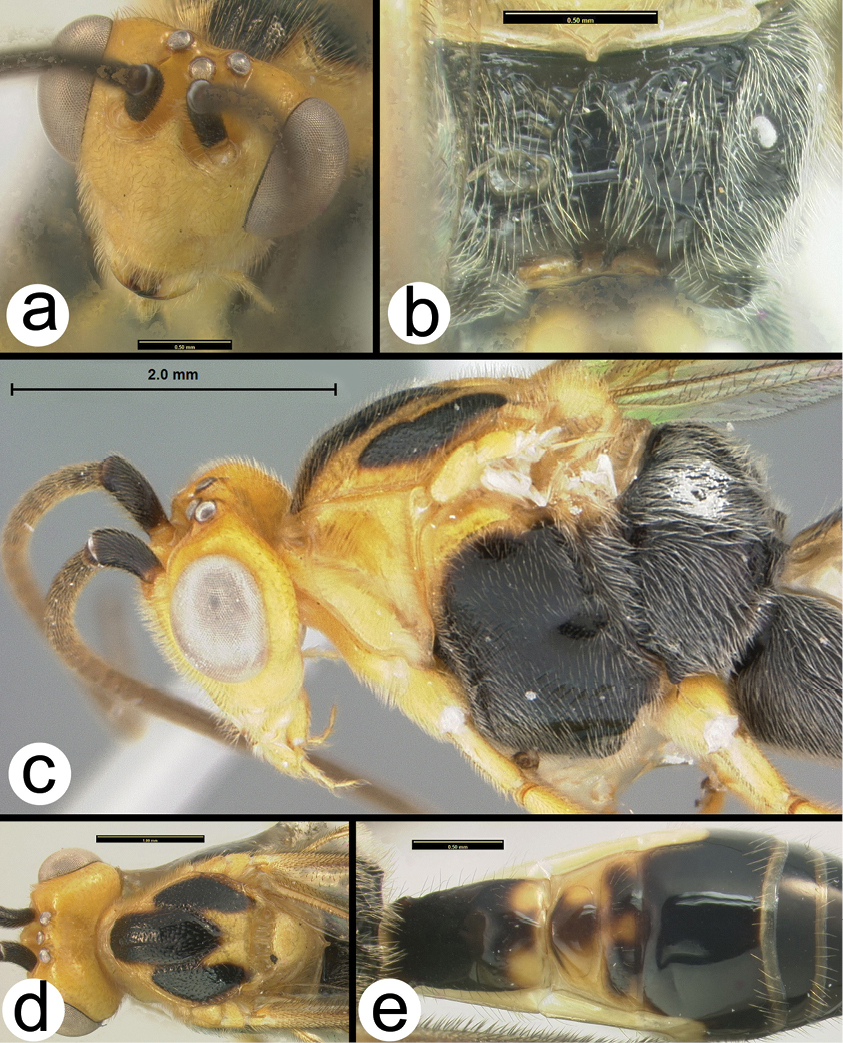
OOL = ocellar ocular length; LOD = lateral ocellar diameter; IOL = inter ocellar length; EH = eye height; MS = malar space length. Measurements are given for the length and apical width of the first metasomal median tergite. Measurement of the apical width is straightforward, however since the base of the tergite is usually hidden from view it is difficult to measure the total length. Instead we measure from the apex of the large tendon that emanates from the propodeum and inserts near the base of the median tergite. Both measurements are illustrated in figure 27.
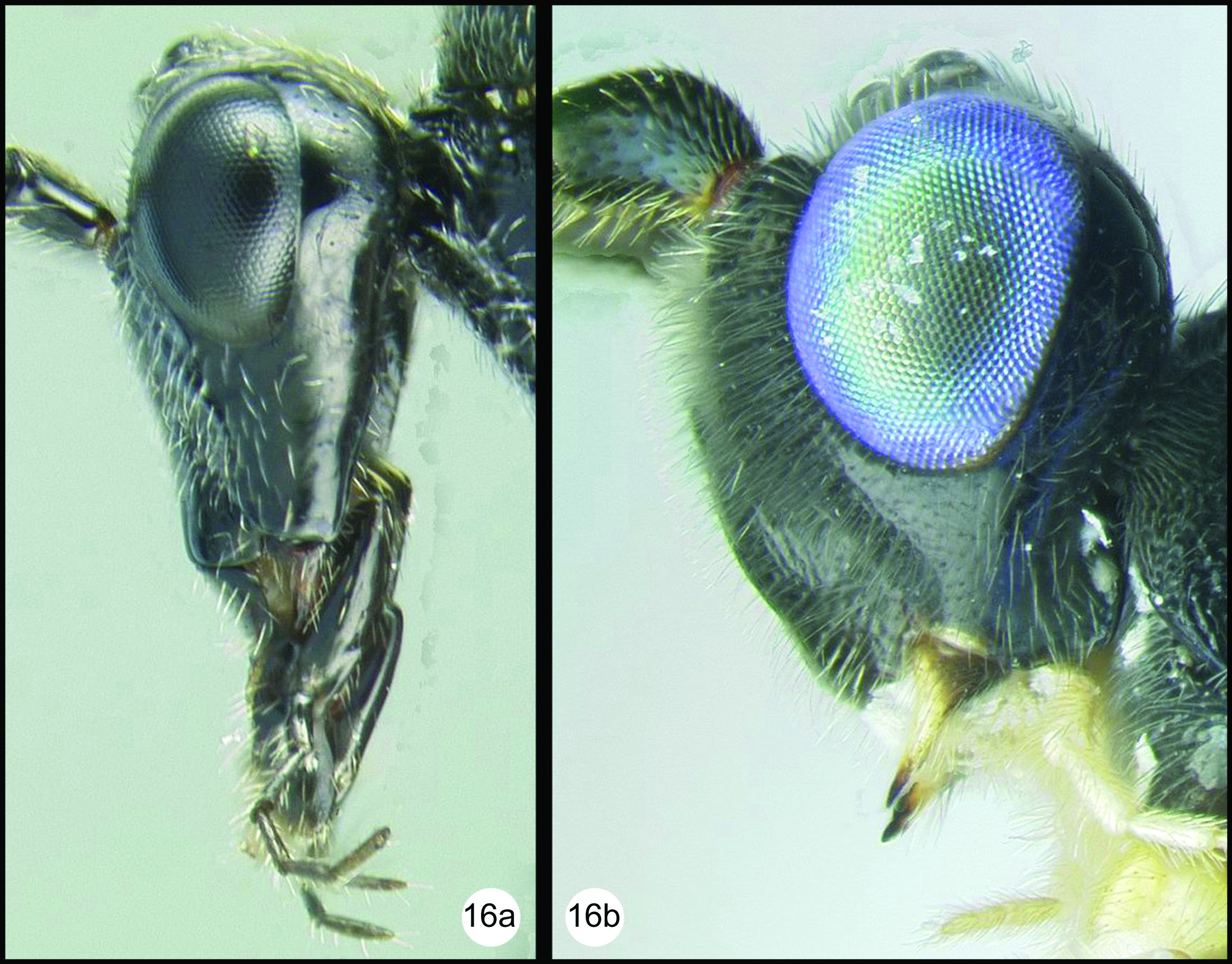
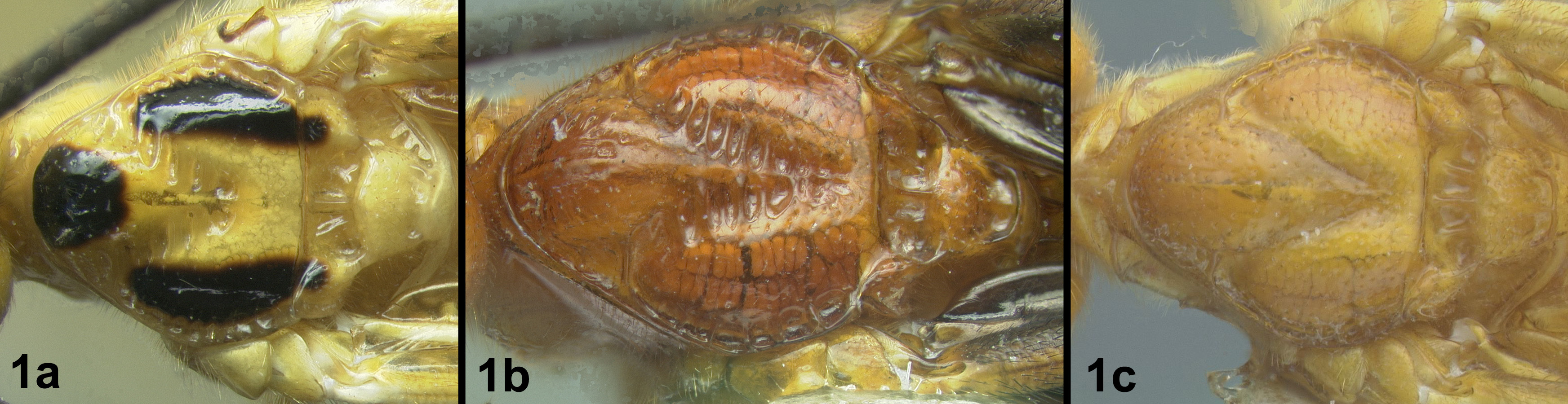
All 25 species are treated with a diagnosis and distribution data. In a few instances a short description is also given for previously described species. The six new species are described with little text; however they, and most previously described species, are comprehensively illustrated with color photos using a JVC digital camera mounted on a Leica MZ16 microscope and Automontage® stacking software. Written descriptions of color patterns are considered redundant due to the completeness of the color illustrations, however when color variation occurs within a species this is referred to in the text, if it is not illustrated. It has been common practice to carefully describe coloration but in our view this is a hold-over from the past when extensive color images were impractical or impossible. The color information is much more accurately recovered from the color photos. Tedious measurements and details of sculpture are not included unless they are of diagnostic value. It is difficult to predict which of the hundreds of possible characters are worth describing and this is rather dependent on the discovery of new species that may be similar to some of those described here. However since all body parts are extensively illustrated and if, for example, the sculpture of the metapleuron or the length of the malar space is important to distinguish a new species, this information is contained in the illustrations. Distribution data are listed for all new species and a Google map via Berkeley Mapper is included for all species.
Distribution data, pdf’s of non-copywrite references, images, notes, and host and type information can be found by searching TaxaBank (a combined specimen and taxonomic database developed by Dr. Dicky Yu in the Sharkey lab; http://purl.org/taxabank) using the specimen voucher H number.
Abbreviations. Abbreviations used for institutions for where specimens are deposited are as follows:
AEI American Entomological Institute, Gainesville, Florida, USA.
BMNH Natural History Museum, London, England.
CNC Canadian National Collection of Insects, Ottawa, Ontario, Canada.
FSCA Florida State Collection of Arthropods, Gainesville, Florida, USA.
HIC Hymenoptera Institute Collection, University of Kentucky, Department of Entomology, Lexington, Kentucky, USA.
HNHM Hungarian Natural History Museum, Budapest, Hungary.
IZCAS Chinese Academy of Sciences, Institute of Zoology, Beijing, China.
MNHN Museum National d’Histoire Naturelle, Paris, France.
MZPW Polish Academy of Science, Museum of the Institute of Zoology, Warsaw, Poland.
NHRS Naturhistoriska riksmuseet, Stockholm, Sweden.
OUMNH University Museum of Natural History, Oxford, United Kingdom QSBG Queen Sirikit Botanic Garden, Chaing Mai. Thailand.
RMNH NCB Naturalis Collection [formerly Rijksmuseum van Natuurlijke Historie], Leiden, Netherlands.
UKM Universiti Kebangsaan, Bangi, Selangor, Malaysia.
USNM National Museum of Natural History, Smithsonian Institution, Washington DC, USA.
Taxonomy Key to Thai genera of AgathidinaeThe key of
| 1 | Fore wing venation greatly reduced, last abscissa (segment) of RS vein completely absent (1a) | Aneurobracon Brues, 1930 |
| – | Fore wing venation more complete, last abscissa of RS vein present though sometimes weak (1b) | 2 |
| 2(1) | Fore and mid claws cleft (2a) | 3 |
| – | Fore and mid claws with a basal lobe (2b) | 12 |
| – | Fore and mid claws simple (2c) | Bassus Fabricius, 1804 |
| 3(2) | Notauli absent (3a) | 4 |
| – | Notauli present but not necessarily complete (3b) | 5 |
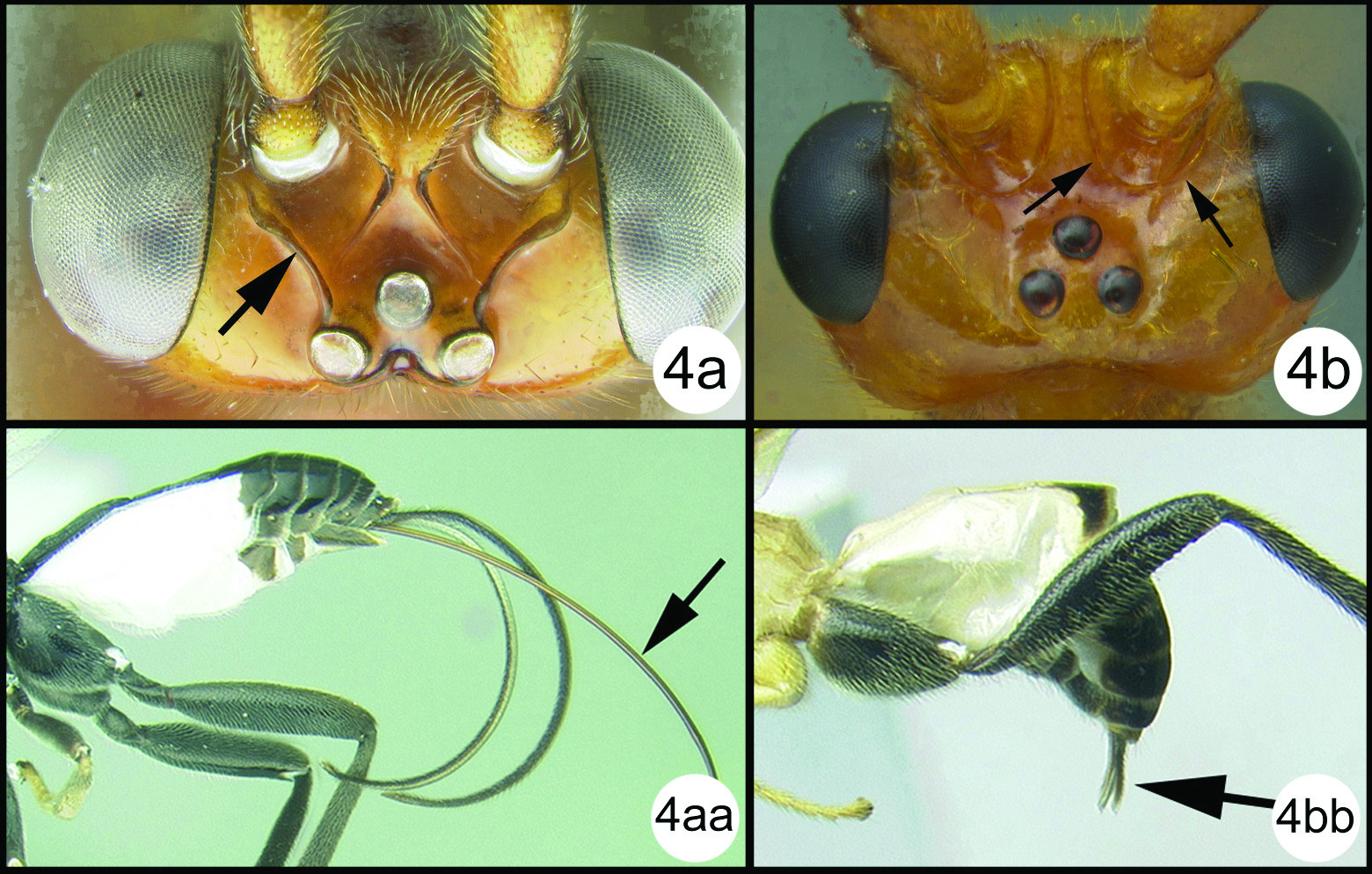
| 4(3) | Lateral carina of frons directed towards lateral ocellus and not fused with medial carina (4a); ovipositor at least as long as metasoma (as in 4aa) | Biroia Szépligeti, 1900 |
| – | Lateral carina of frons fused with medial carina forming a circular border around antennal base (4b); ovipositor barely exerted (as in 4bb) | Gyrochus Enderlein, 1920 |
| 5(3) | Hind trochantellus with one or two distinct carinae (5a) | 6 |
| – | Hind trochantellus lacking carinae (5b) | 9 |
| 6(5) | Fore tibial spurs about as long as basitarsus and ending in a long thin style (6a) | Coccygidium Saussure, 1892 |
| – | Fore tibial spurs less that 3/4 length of fore basitarsus and ending abruptly (6b) | 7 |
| 7(6) | Posterolateral margins of frons bordered with carinae (7a) | 8 |
| – | Posterolateral margins of frons not bordered with carinae (7b) | Zelodia van Achterberg & Long, 2010 |
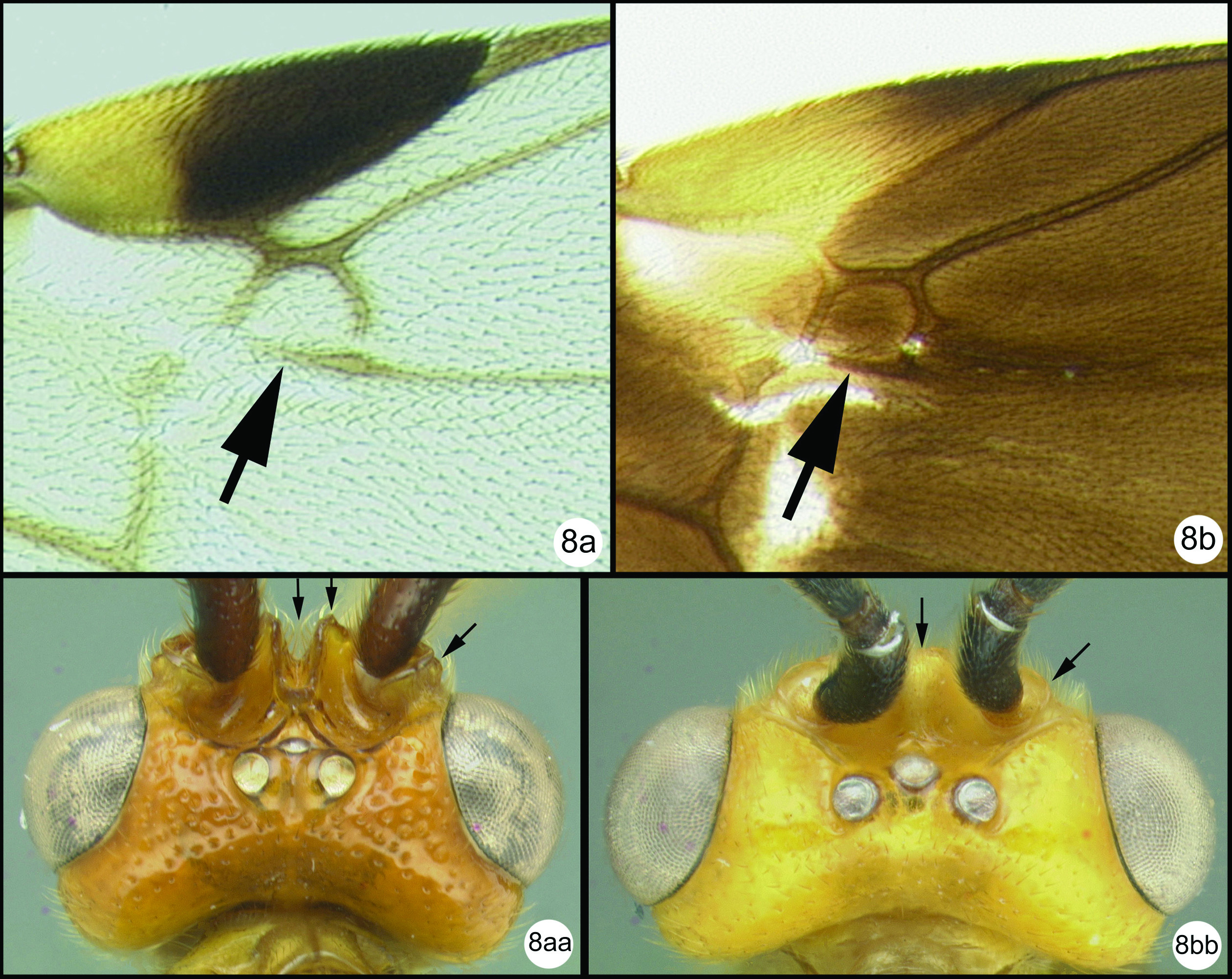
| 8(7) | 2nd submarginal cell of fore wing triangular or distinctly narrowing anteriorly (8a); base of antenna surrounded by pronounced medial, lateral, and anterior ridges; deep groove between antennae (8aa) | Hypsostypos Baltazar, 1963* |
| – | 2nd submarginal cell of fore wing quadrate, not distinctly narrowing anteriorly (8b); base of antenna surrounded by weak lateral, and anterior ridges; lacking deep groove between antennae (8bb) | Cremnoptoides van Achterberg & Chen, 2004 |
| 9(5) | Posterolateral margins of frons not bordered with carinae (9a) | Euagathis Szépligeti, 1900 |
| – | Posterolateral margins of frons bordered with carinae (9b) | 10 |
| 10(9) | Medial and lateral carinae of frons lamellate (high and thin) (10a); ovipositor barely exerted, much shorter than half length of metasoma (see 4bb) | 11 |
| – | Medial and lateral carina of frons in the form of blunt ridges, not lamellate (10b); ovipositor at least as long as the metasoma (see 4aa) | Cremnops Förster, 1862 |
| 11(10) | Lateral carina of frons with posterior ends directed towards median ocellus (11a) | Troticus Brullé, 1846 |
| – | Lateral carina of frons with posterior ends directed towards lateral ocelli (11b) | Disophrys Förster, 1862 |
| 12(2) | RS+M vein of fore wing mostly or entirely absent (12a); notauli present but not necessarily complete (12aa) | 13 |
| – | RS+M vein of fore wing present and complete (12b); notauli absent (12bb) | Earinus Wesmael, 1837 |
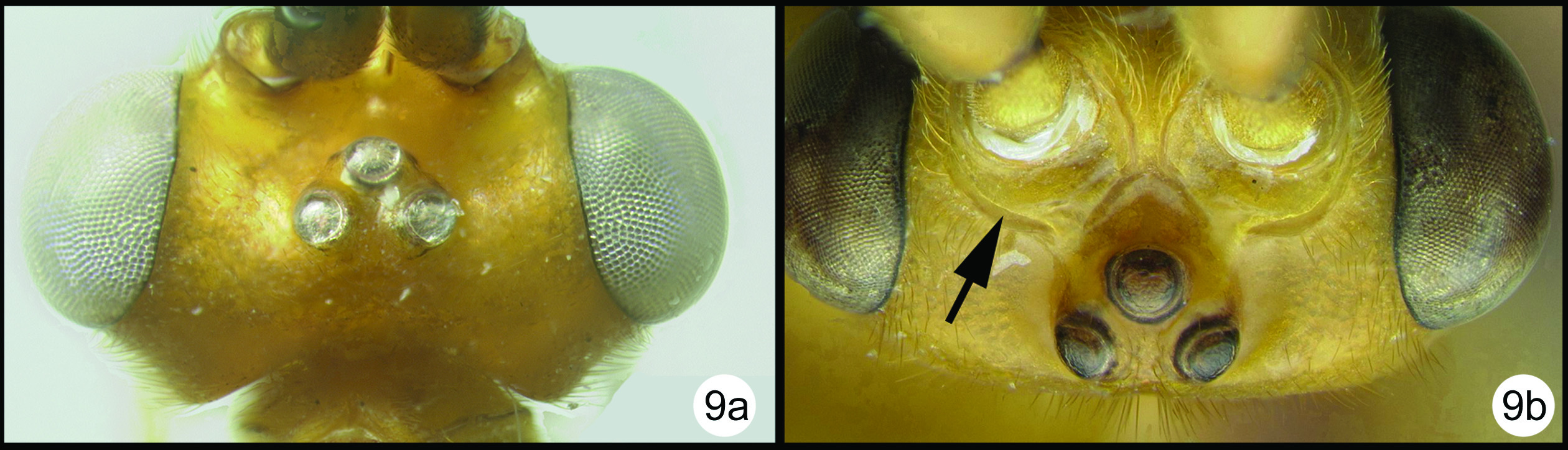
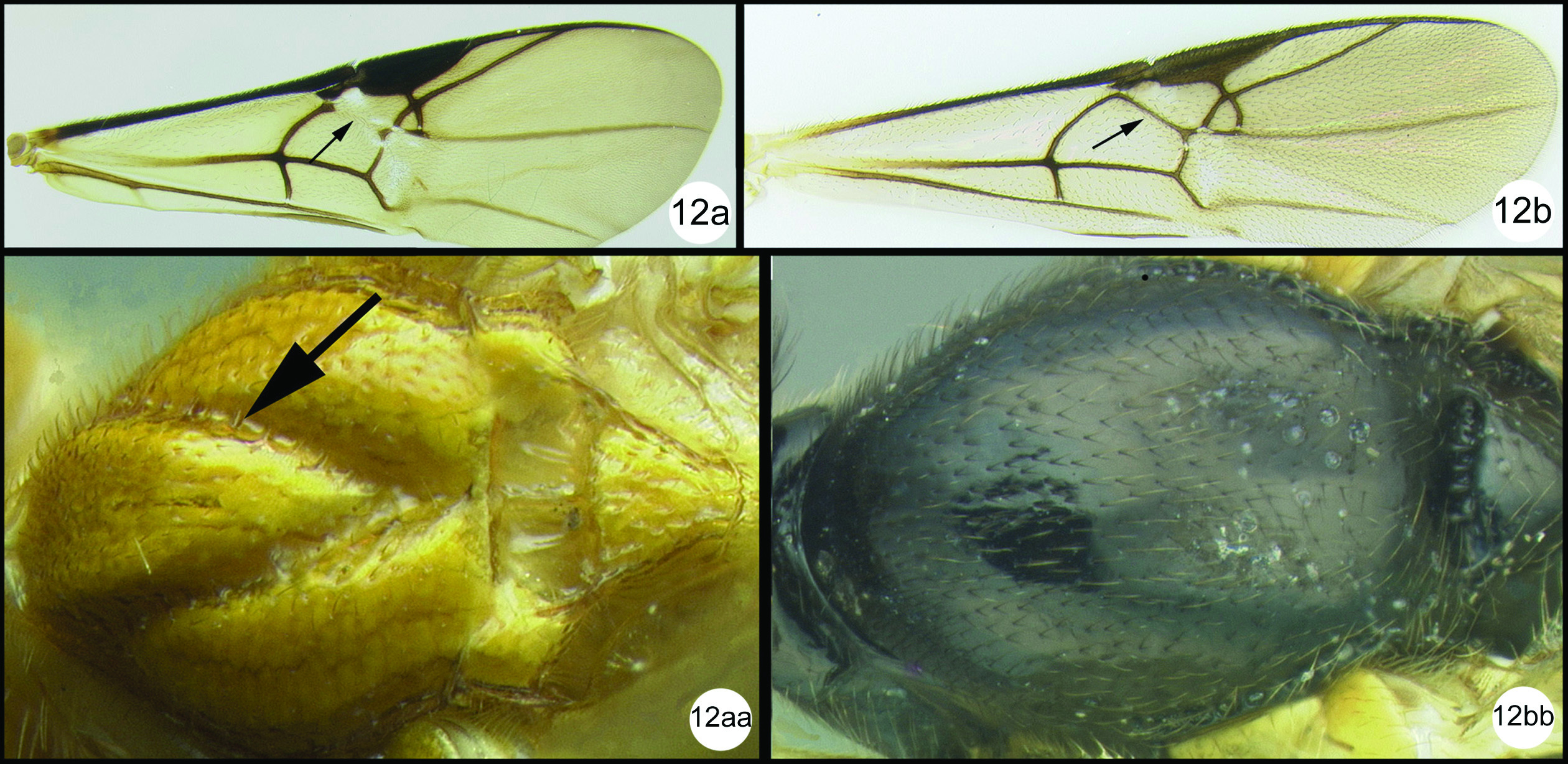
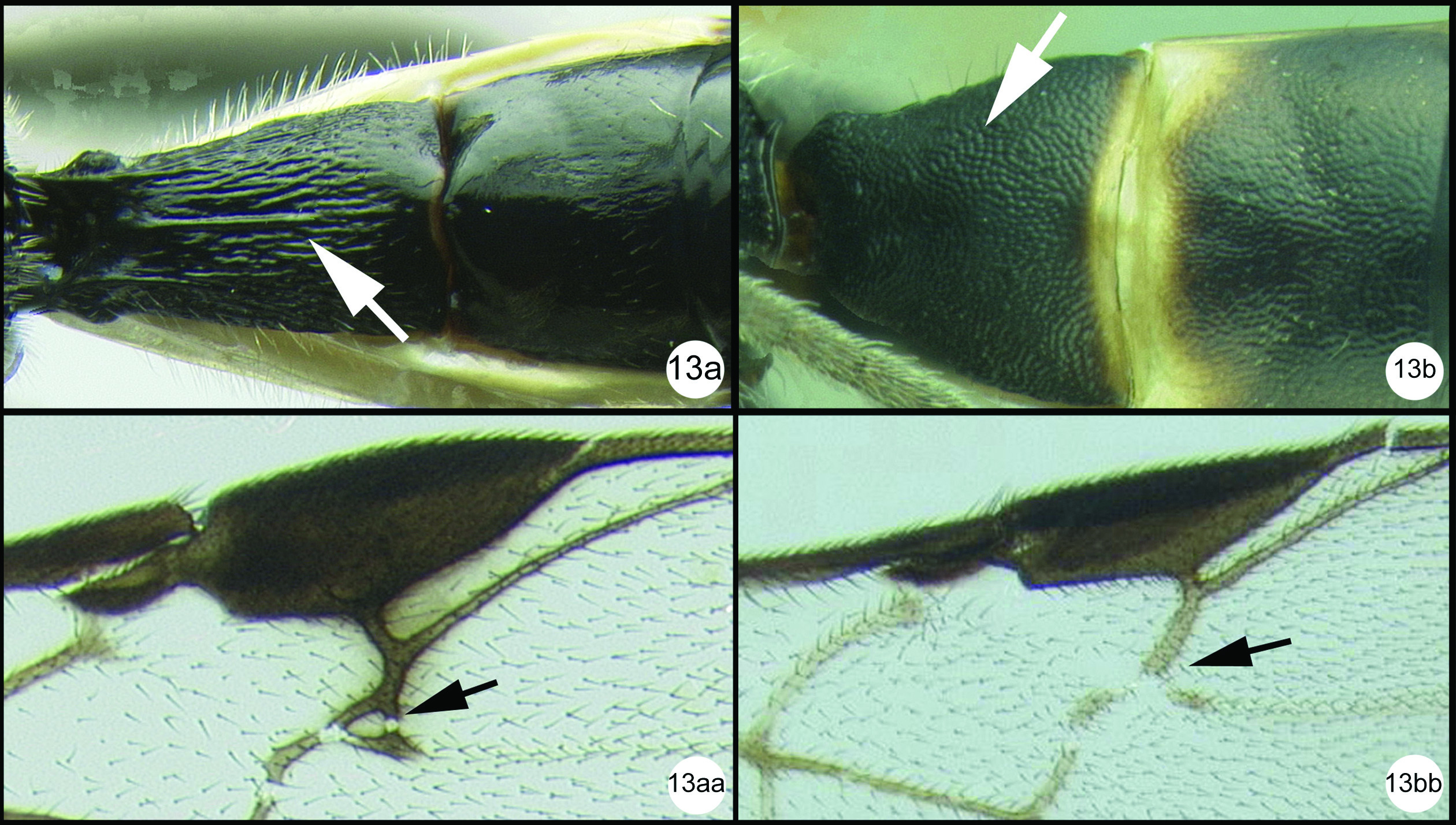
| 13(12) | First median tergite mostly striate (13a) or (rarely) smooth; 2nd submarginal cell of fore wing usually present (13aa) | 14 |
| – | First median tergite mostly granulate or coriaceous (13b); 2nd submarginal cell of fore wing absent (13bb) | Camptothlipsis Enderlein, 1920 |
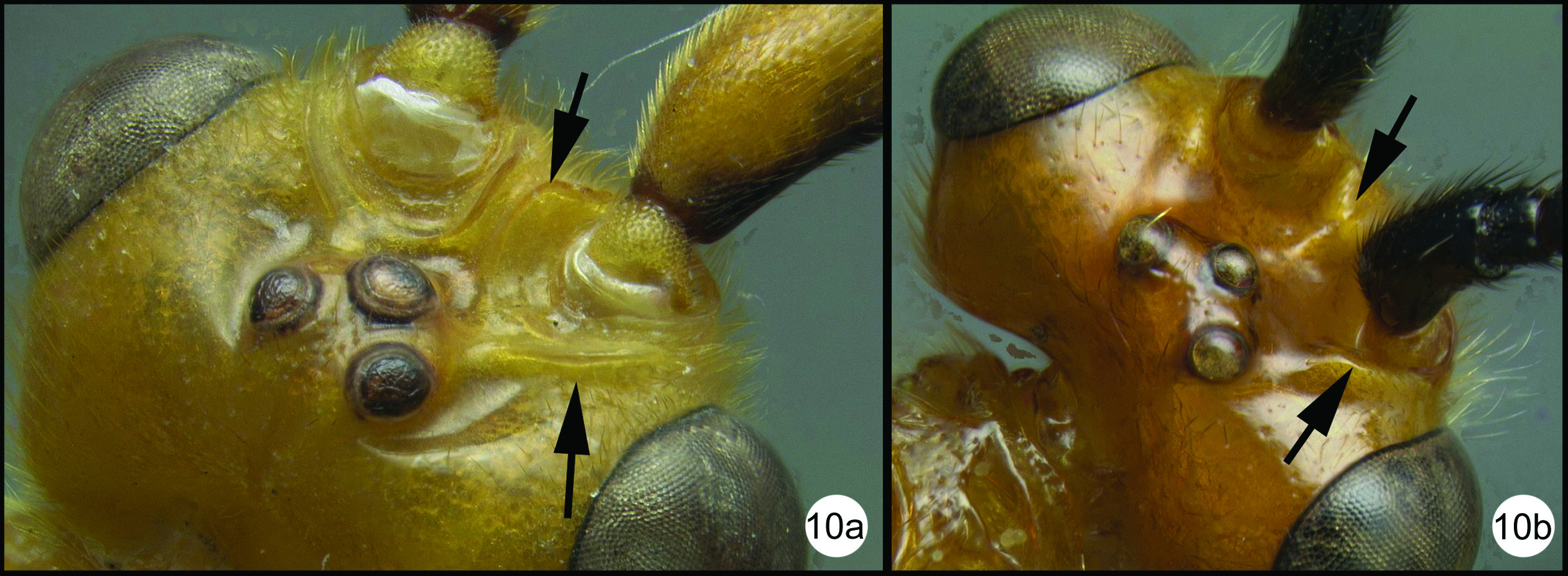
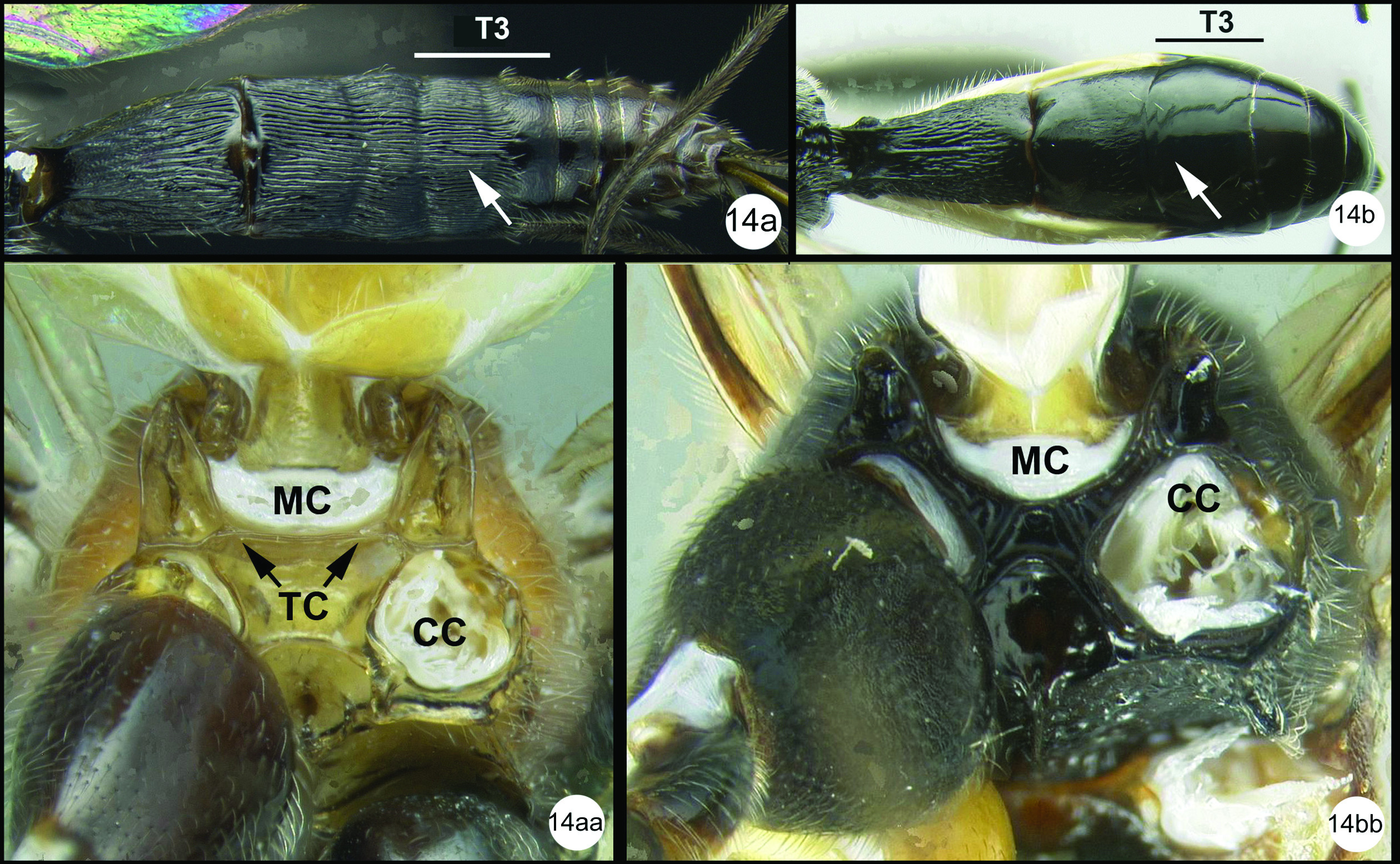
| 14(13) | Median tergite 3 extensively striate in anterior half or more (14a); metasomal cavity (MC) situated entirely dorsal to coxal cavities (CC); a wide, high, straight, transverse carina (TC) present between metasomal cavity (MC) and coxal cavities (CC) (14aa) | 15 |
| – | Median tergite 3 smooth (14b); metasomal cavity (MC) situated partly between coxal cavities (CC); wide, high, straight, transverse carina (TC) between metasomal (MC) and coxal cavities absent, usually curved and relatively shallow if present (14bb) | 16 |
| 15(14) | Adventitious vein (2RS) on r-m crossvein of fore wing absent or indicated only by slight swelling (15a) | Lytopylus Förster, 1862 |
| – | Adventitious vein (2RS) on r-m crossvein of fore wing present and distinct (15b) | Braunsia Kriechbaumer, 1894 |
| 16(14) | Mouthparts long, galea significantly longer than wide; gena usually elongate (16a) | Agathis Latreille, 1804 (not yet discovered in Thailand but likely to occur). |
| – | Mouthparts short (normal), galea not longer than wide; gena not elongate (16b) | Therophilus Wesmael, 1837 |
http://species-id.net/wiki/Biroia_fuscicornis
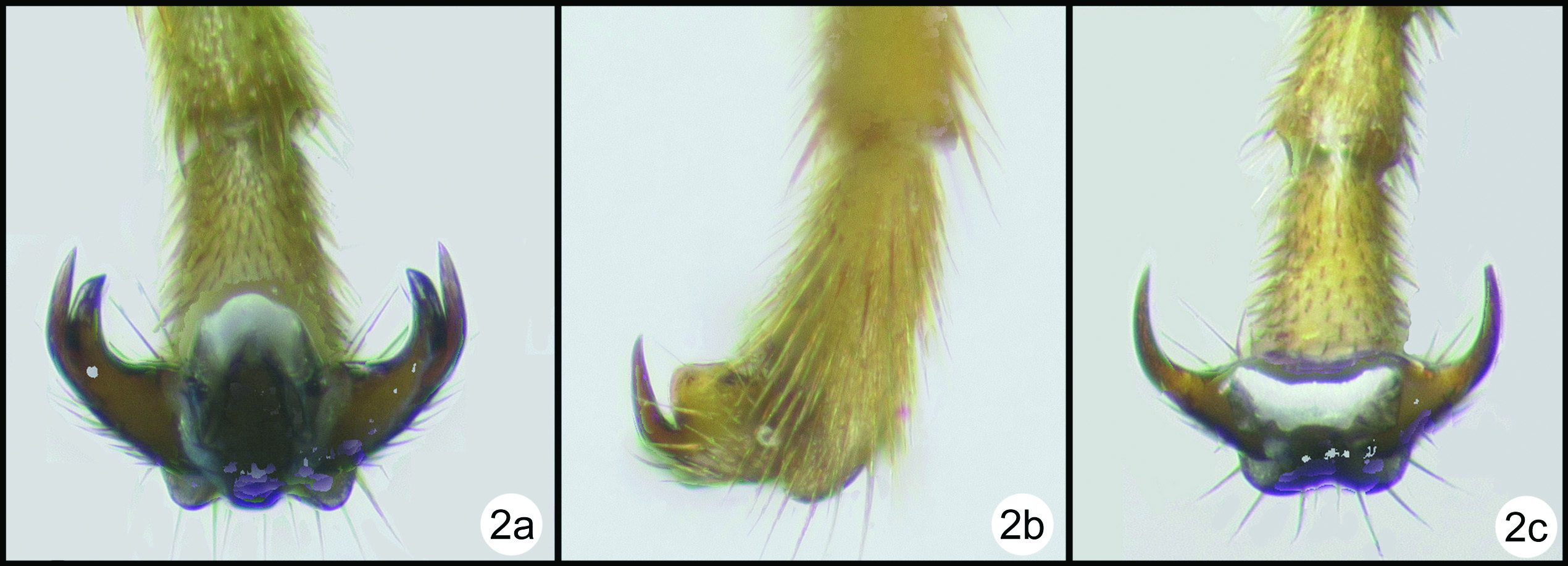
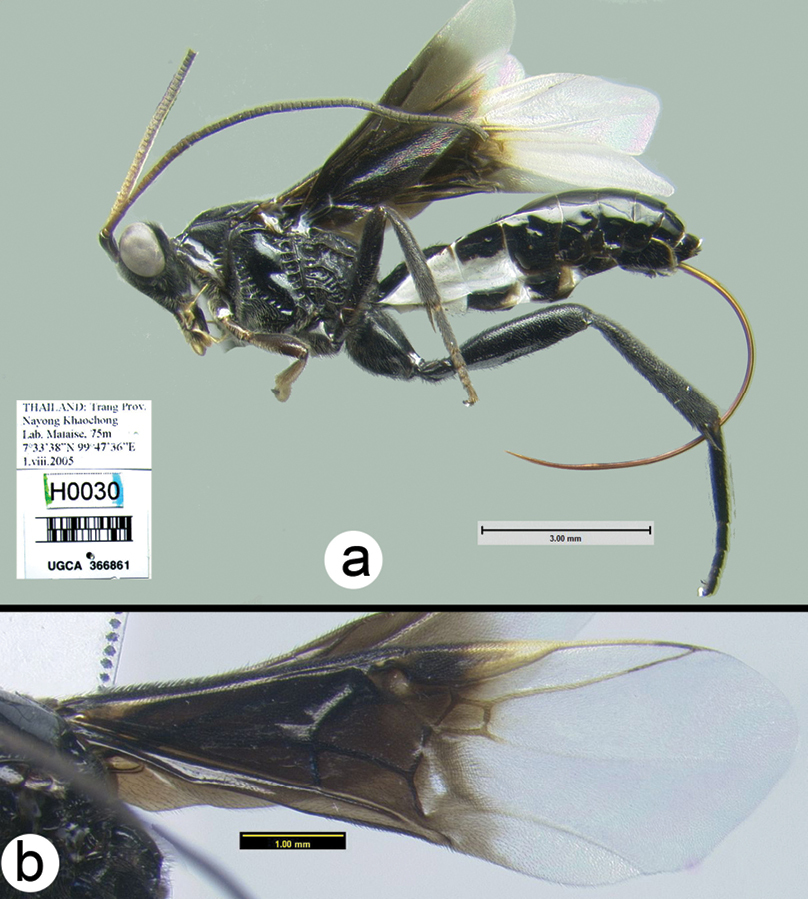
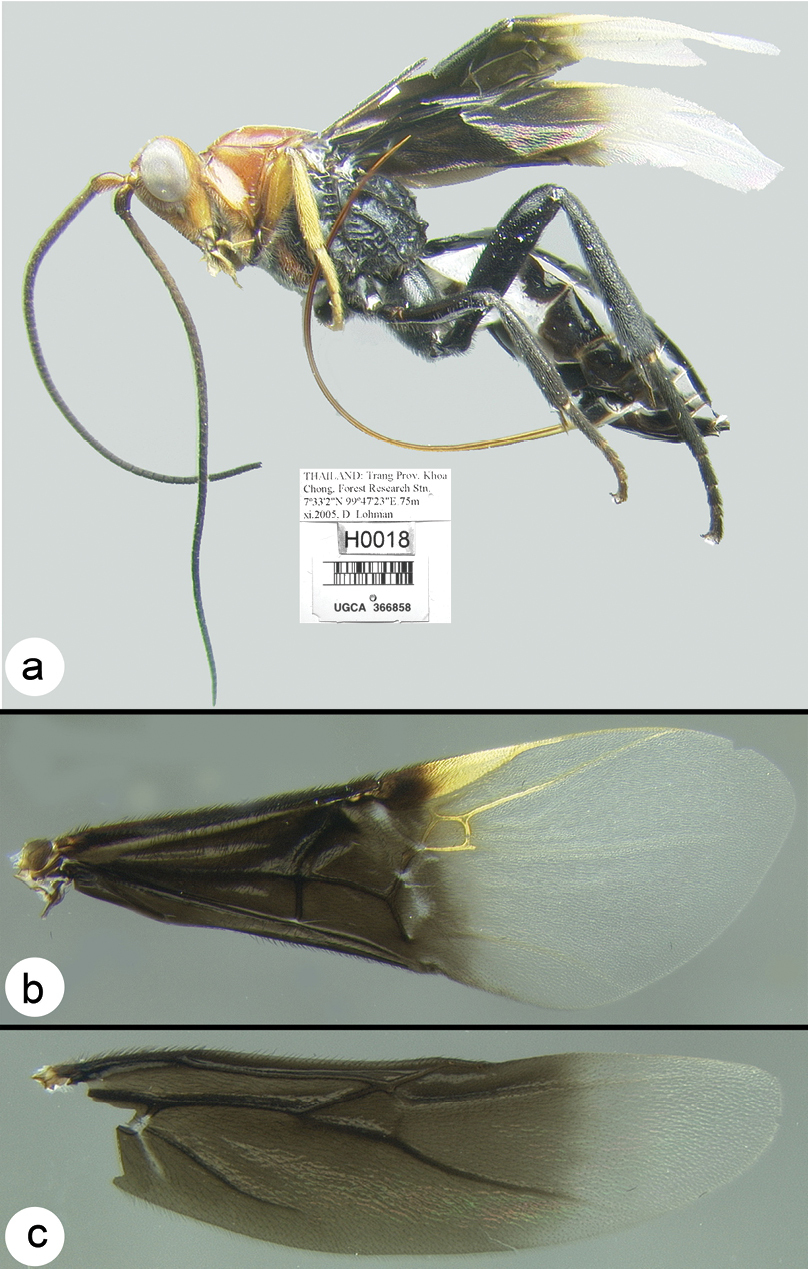
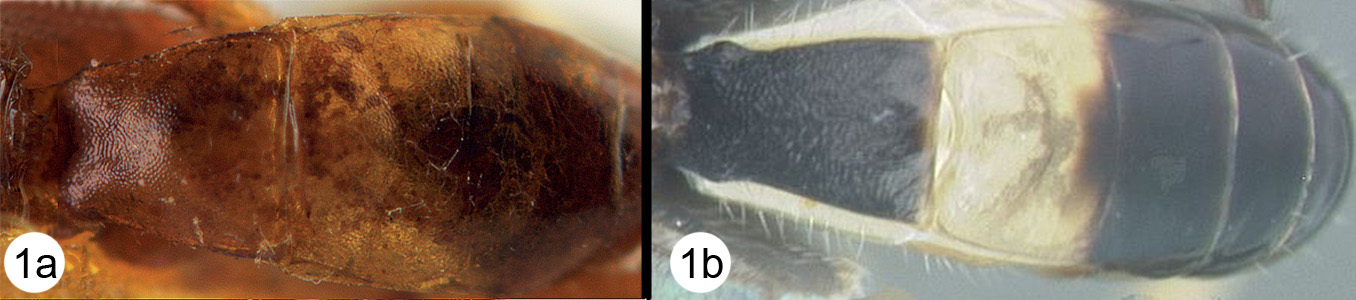
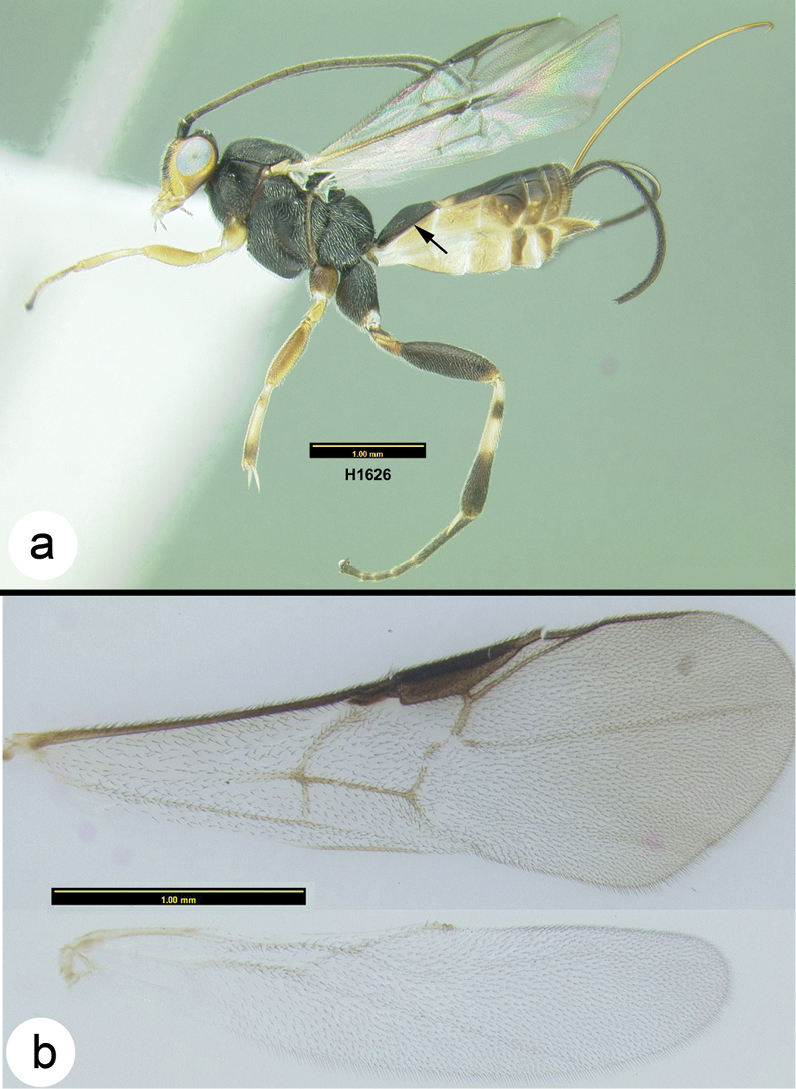
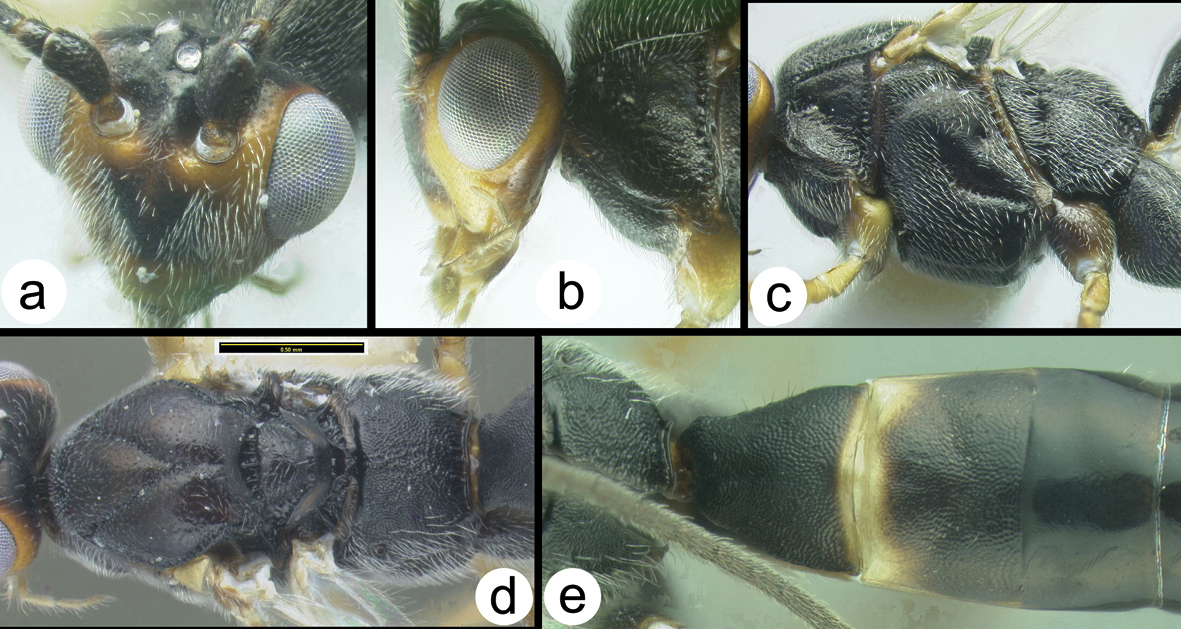
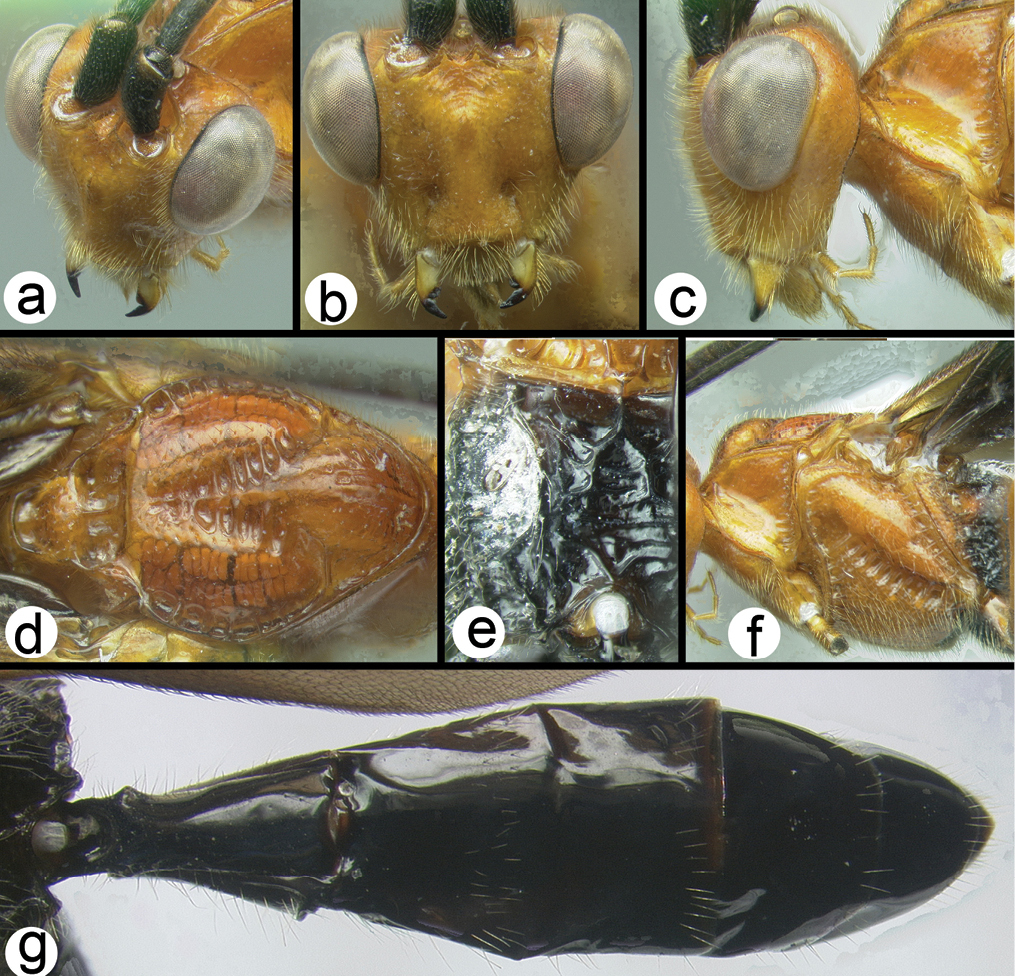
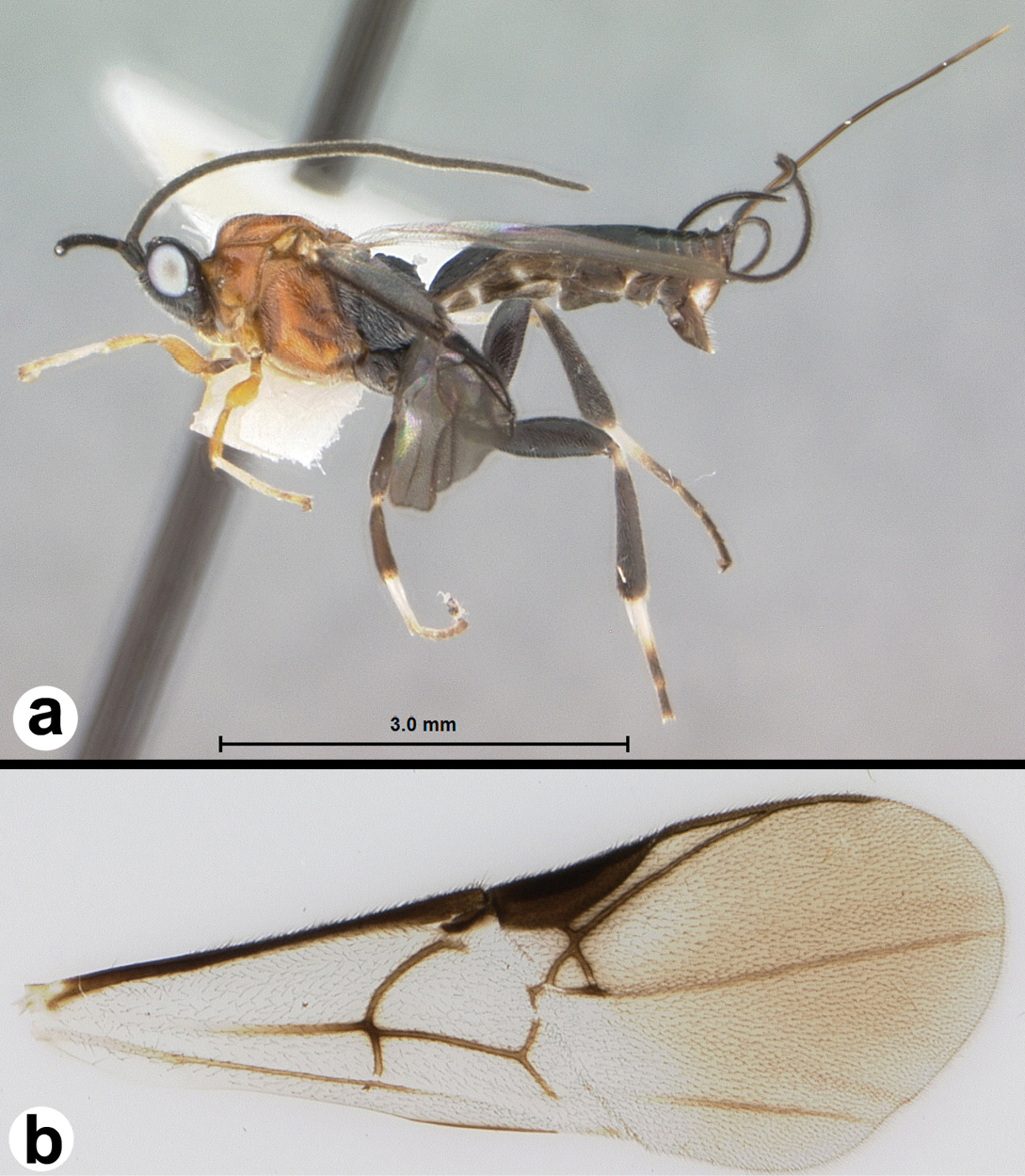
Figs 3-5There is only one species of Biroia in Thailand though it is extreme in its color variation. The head and pro- and mesothorax vary from entirely black to orange. In Thailand there is little variation between these two extremes, although several of the orange forms have the vertex black. One specimen from Brunei has the head mostly black and the mesoscutum black in the posterior 2/3. The wings are melanic-infuscate basally and milky white distally. 28S sequence data for four specimens of the melanic form and one specimen of the orange and black form are identical (Fig. 2).
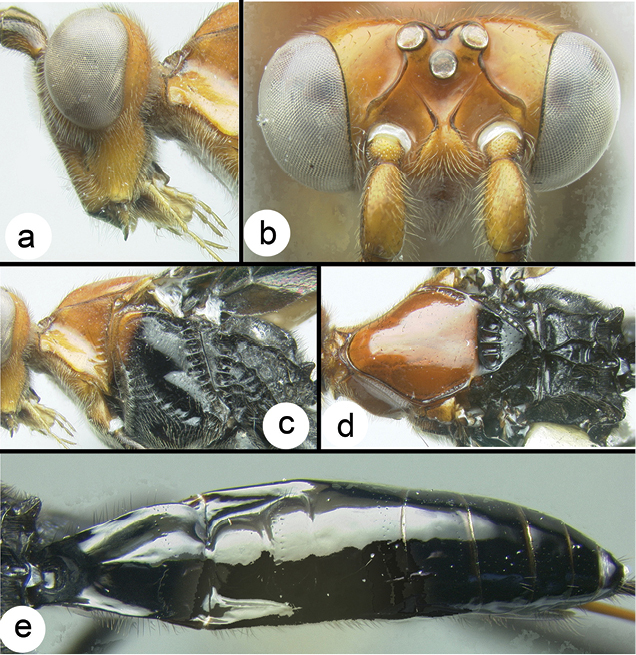
Biroia fuscicornis
Figure 3. Biroia fuscicornis Cameron, dark morph a lateral habitus b fore wing.
Figure 4. Biroia fuscicornis Cameron, light morph a lateral habitus b fore wing c hind wing.
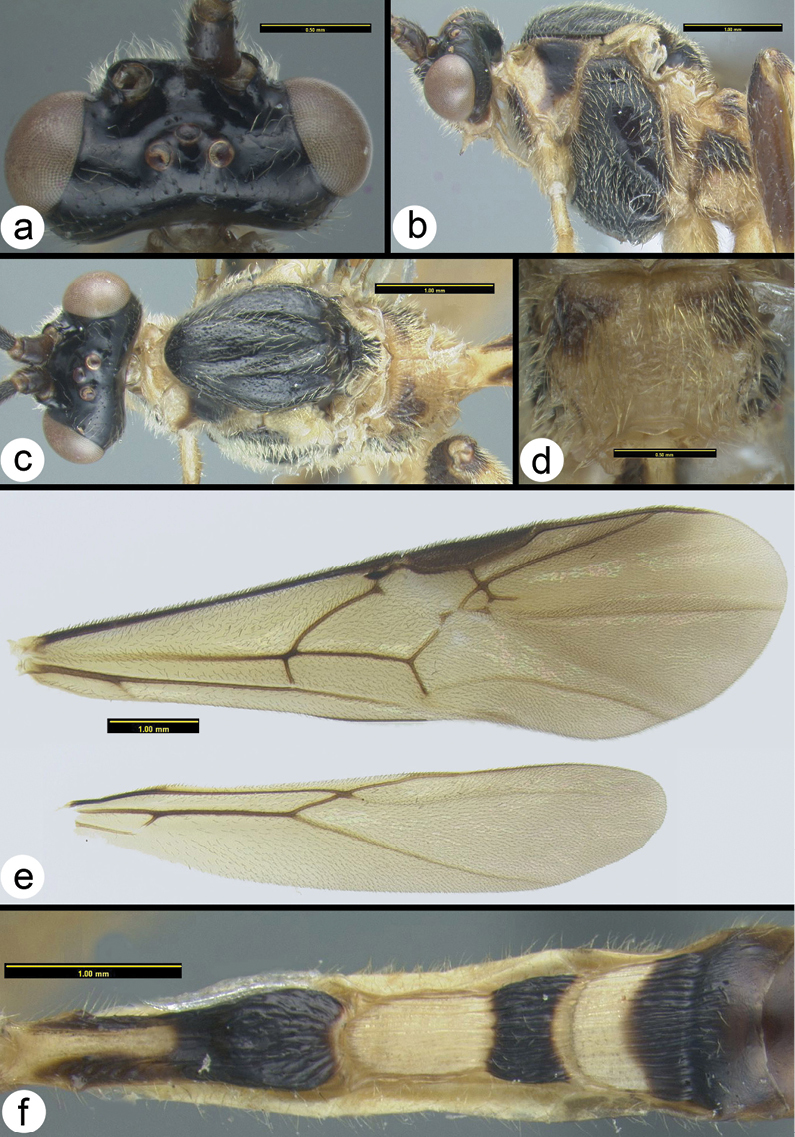
Biroia fuscicornis Cameron, light morph a lateral head b dorsal head c lateral mesosoma d dorsal mesosoma e dorsal metasoma.
H0030: #HQ667945 (black form). H0018: #HQ667942. H0020: #HQ667943. H0049: #HQ667944. H0082: #HQ667941
Peninsular Malaysia, Borneo (Sarawak, Brunei), Indonesia (Sumatra), Singapore, Thailand, Vietnam. Distribution maps can be found at http://purl.org/thaimaps/bfuscicornis.
Examined specimens are deposited in the following collection: MZPW, BMNH, HIC, USNM, UKM, QSBG, RMNH.
Braunsia
Key to Thai species of Braunsia
Only two species are known from Thailand; however three species from Peninsular Malaysia are included due to their likelihood of occurring in southern Thailand.
| 1a | Dorsal head and mesoscutum entirely yellow | 2 |
| 1b | Dorsal head and mesoscutum entirely reddish brown | Braunsia sumatrana Enderlein |
| 1c | Dorsal head black and mesoscutum melanic or mostly melanic | 3 |
| 1d | Dorsal head black, mesoscutum yellowish orange | Braunsia fumipennis (Cameron) |
| 2a | Apex of antenna yellow, hind tibia with 13 or more spines | Braunsia chaweewanaeSharkey, sp. n. |
| 2b | Apex of antenna melanic, hind tibia with 12 or fewer spines | Braunsia smithiiDalla Torre |
| 3a | Mesosoma with many infusions of yellow color especially on pronotum and propodeum | Braunsia comosa Enderlein |
| 3b | Mesosoma mostly melanic with the lateral lobes of scutellum sometimes deep reddish black; pronotum and propodeum melanic | Braunsia burmensis Bhat & Gupta |
http://species-id.net/wiki/Braunsia_burmensis
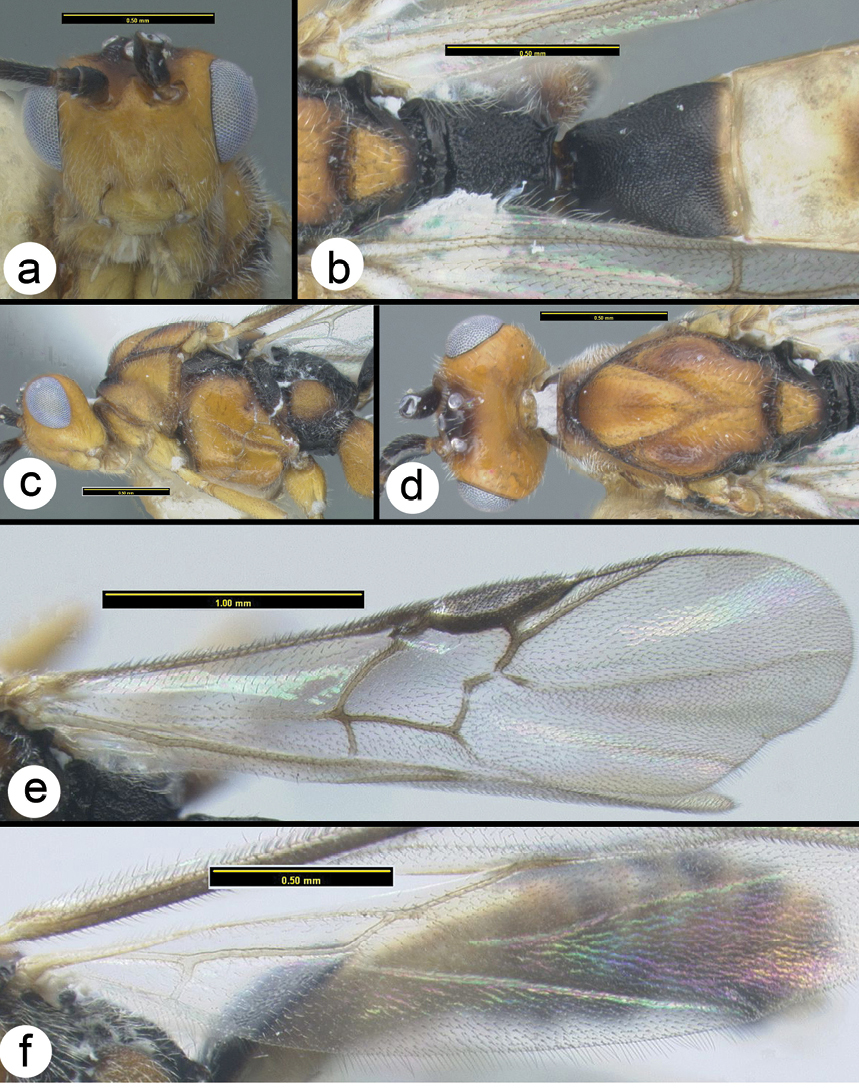
Figs 6Mesosoma entirely melanic, sometimes with the lateral lobes of the mesoscutum reddish black; wings weakly infuscate; second metasomal median tergite pale in basal half or more.
Figure 6. Braunsia burmensis Bhat & Gupta a lateral habitus b dorsal habitus c fore wing.
H1101: #DQ201930
Myanmar and Peninsular Malaysia (Cameron Highlands). Specimens are deposited in NHRS (holo- and allotype), and HIC (one specimen). The species is included here due to its likelihood of occurring in Thailand. Distribution maps can be found at http://purl.org/thaimaps/burmensis.
urn:lsid:zoobank.org:act:71694C50-E347-46B6-86E4-DDA3FCC668CD
http://species-id.net/wiki/Braunsia_chaweewanae
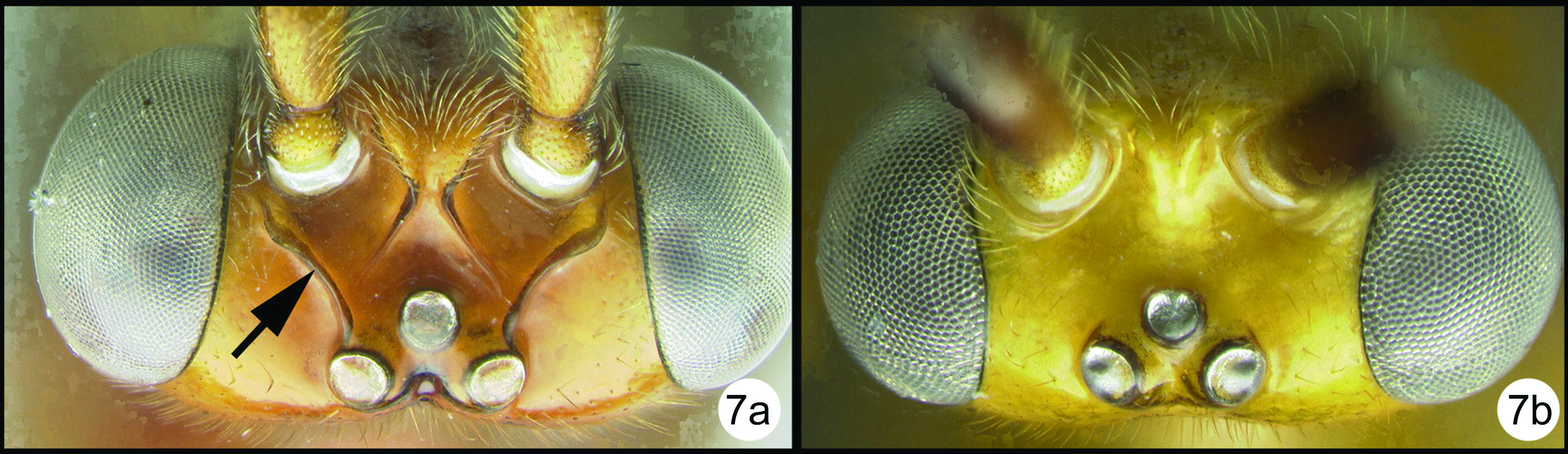
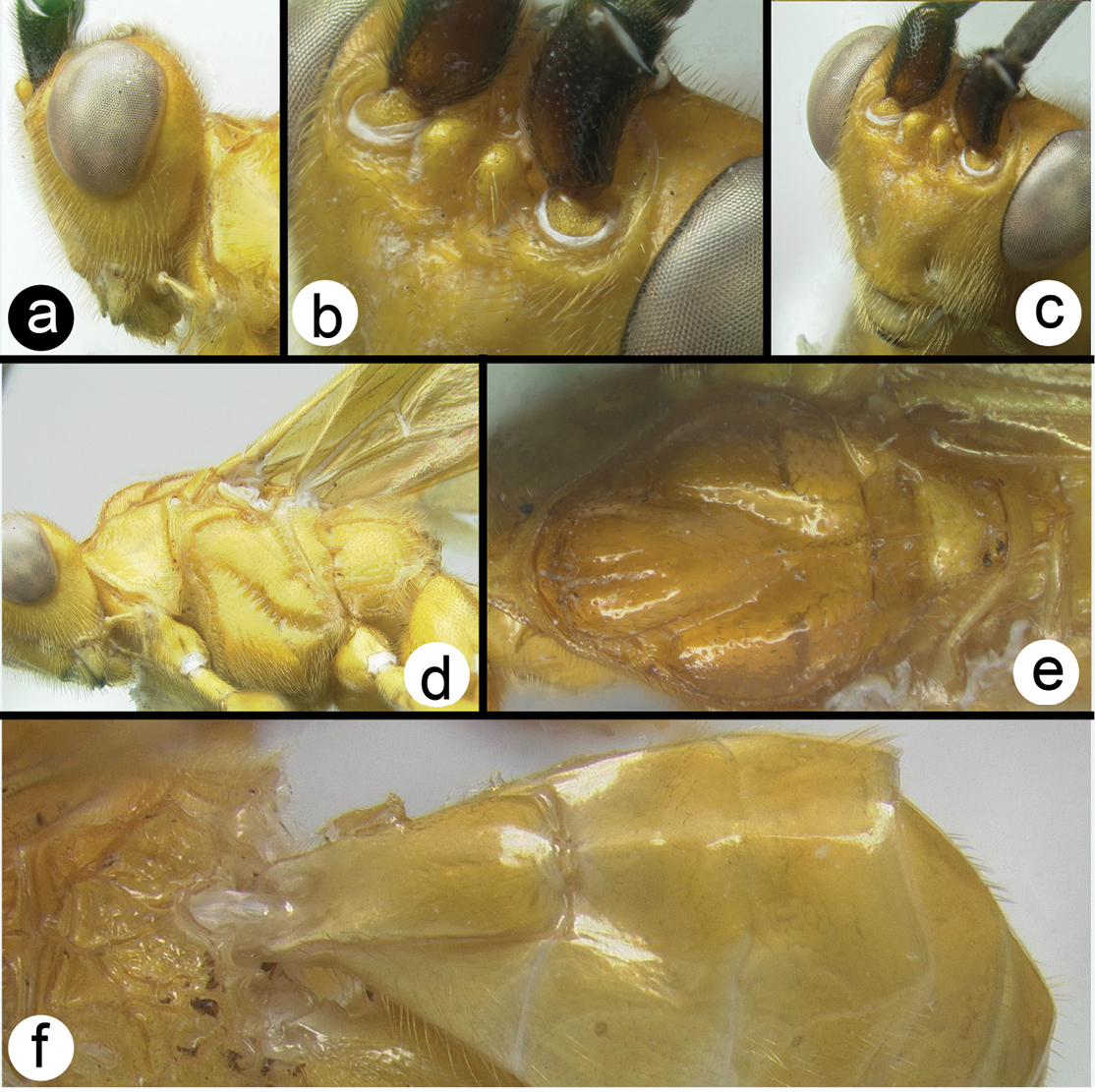
Figs 7–8Differs from Braunsia smithii (
Holotype female.Essentially as in Braunsia smithii except as follows:
Body length. 9.5mm.
Head. OOL 0.32; POD 0.13; IOL 0.16; 45 flagellomeres
Mesosoma. tubular portion of 2RS2 of fore wing shorter than length of 2nd submarginal cell; midtibia with 9 (right) to 11 (left) melanic spines; hind tibia with 16 (right) spines.
Metasoma. Median tergite 1 about 1.7X as long as wide apically (length = 1.66mm, width = 1.0mm).
Color. Yellow except as follows: the following are melanic: flagellum except apical segments, large parastigmal spot, distal 1/3 of fore wing and hind wing, apex of hind tibia and tarsus somewhat darkened.
Male. Unknown.
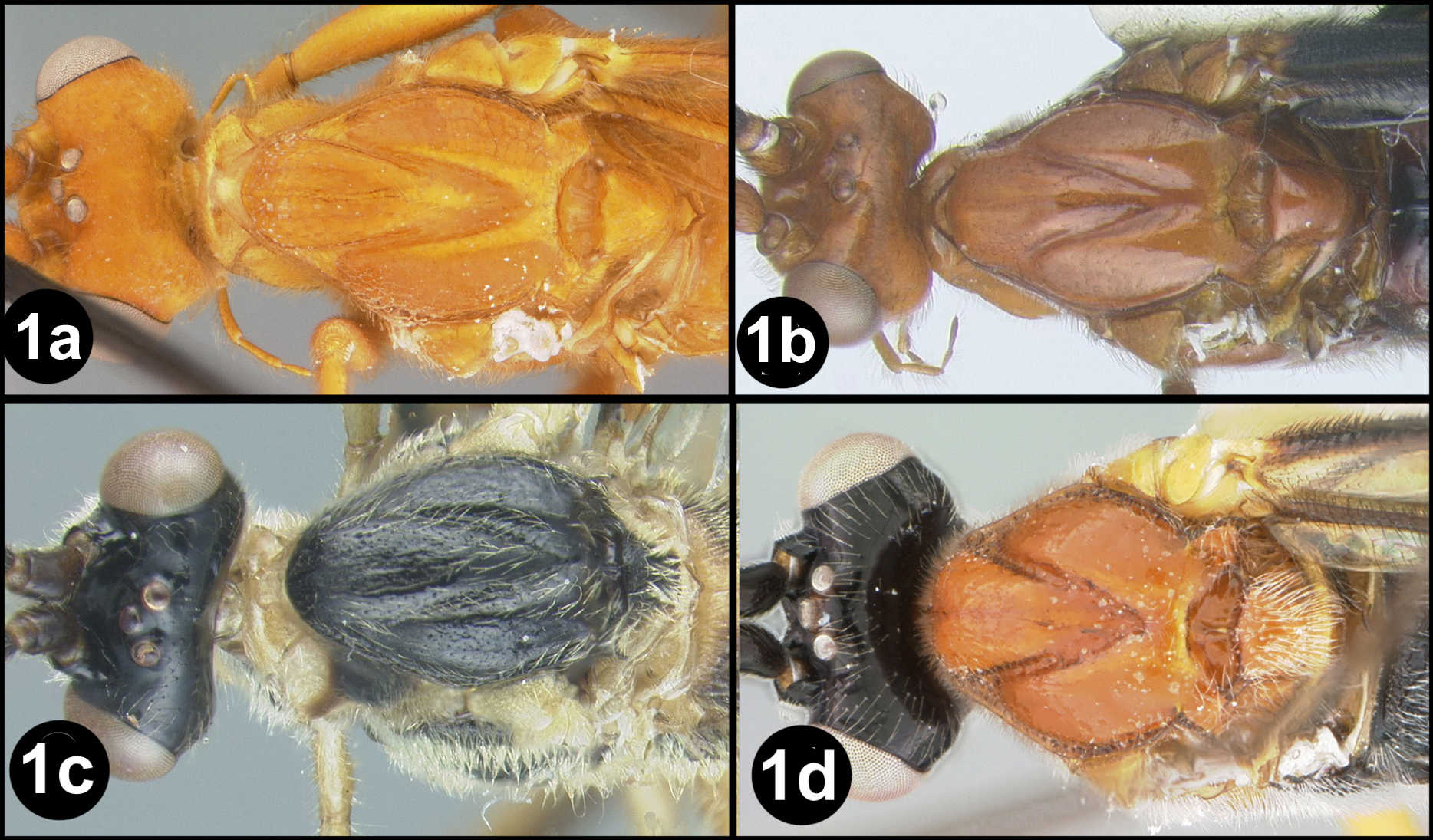
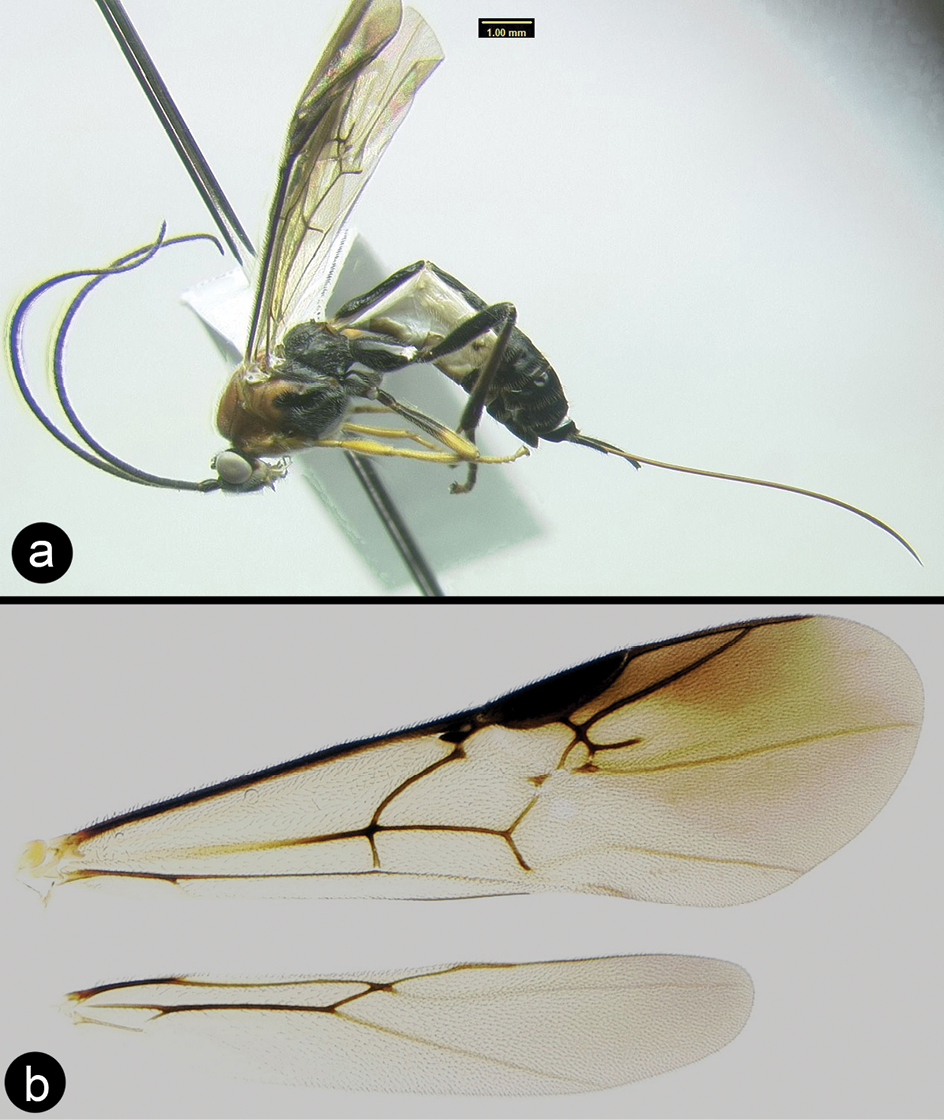
Braunsia chaweewanae sp. n. a lateral habitus b fore wing c hind wing.
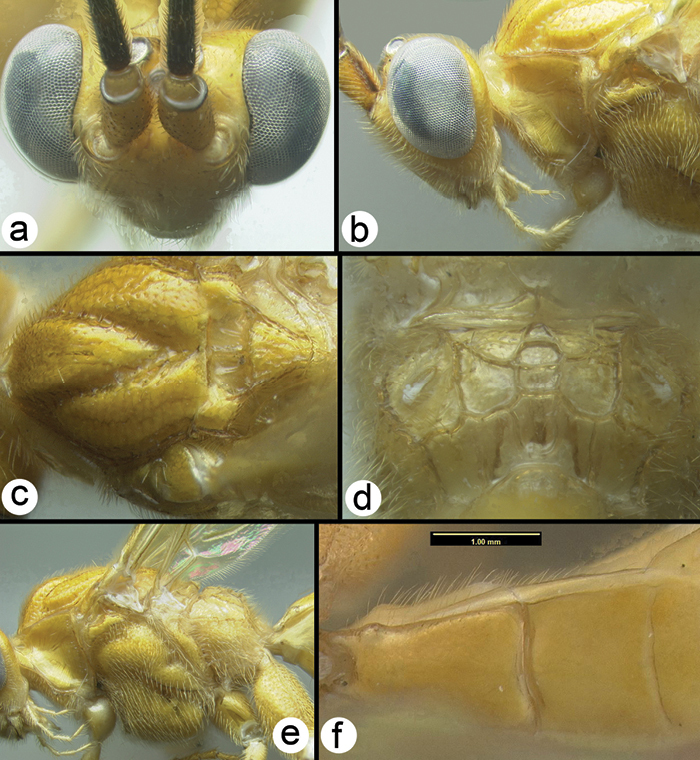
Braunsia chaweewanae sp. n. a dorsal habitus b antenna c dorsal head and mesosoma d dorsal propodeum and metasomal terga 1–3 e lateral head and mesosoma f fore wing showing short vein 2RS2 g hind tibia showing lateral spines.
H245: #HQ667950
Known only from the holotype female from Thailand. Distribution maps can be found at http://purl.org/thaimaps/chaweewanae.
Holotype female: H245, Thailand, Loei, Phu Ruea NP, Suan hin Palee, 17.4977°N, 101.3425°E, 1178m, MT, 12–19.x.2006, Nukoonchai Jaroenchai [QSBG].
http://species-id.net/wiki/Braunsia_comosa
Figs 9– 10The color of this species is unique in that the mesosoma is yellow and black with most of the central areas of the sclerites black and the margins yellow, and the propodeum is mostly yellow. The first metasomal median tergite is long and narrow. The mesosoma, especially the propodeum laterally, is quite setose.
Male. Unknown.
Braunsia comosa Enderlein a lateral habitus b dorsal habitus.
Braunsia comosa Enderlein a dorsal head b lateral mesosoma c dorsal head and mesosoma d dorsal propodeum e wings f dorsal metasomal terga 1–3.
Besides the holotype from Indonesia (Sumatra) one specimen from Peninsular Malaysia is deposited in UKM. Distribution map can be found at http://purl.org/thaimaps/comosa. It is included here due to its likelihood of being present in southern Thailand.
http://species-id.net/wiki/Braunsia_fumipennis
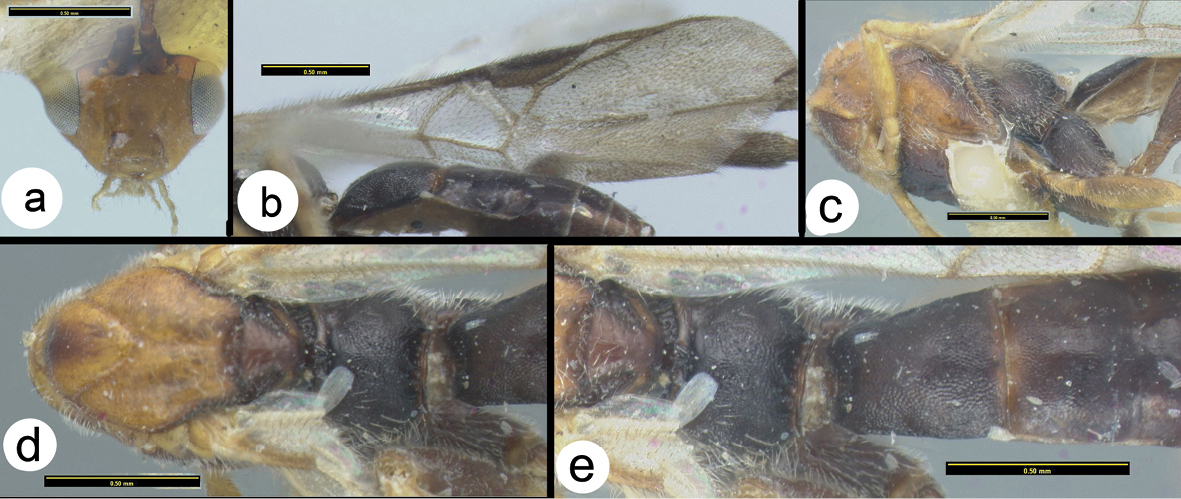
Figs 11–12The color pattern is somewhat similar to that of Braunsia sumatrana, but this is the only similarity between the species. The first median metasomal tergite of Braunsia fumipennis is much narrower than that of Braunsia sumatrana (compare figures 12f and 16c), and the first metasomal tergum of Braunsia fumipennis is entirely melanic whereas that of Braunsia sumatrana is white basally.
The mid femur varies from yellow to black; the fourth metasomal tergum varies from almost completely smooth to aciculate, and may or may not have several transverse rows of setae. None of these character states are correlated in such a manner as to suggest different species.
Braunsia fumipennis (Cameron) a lateral habitus b wings.
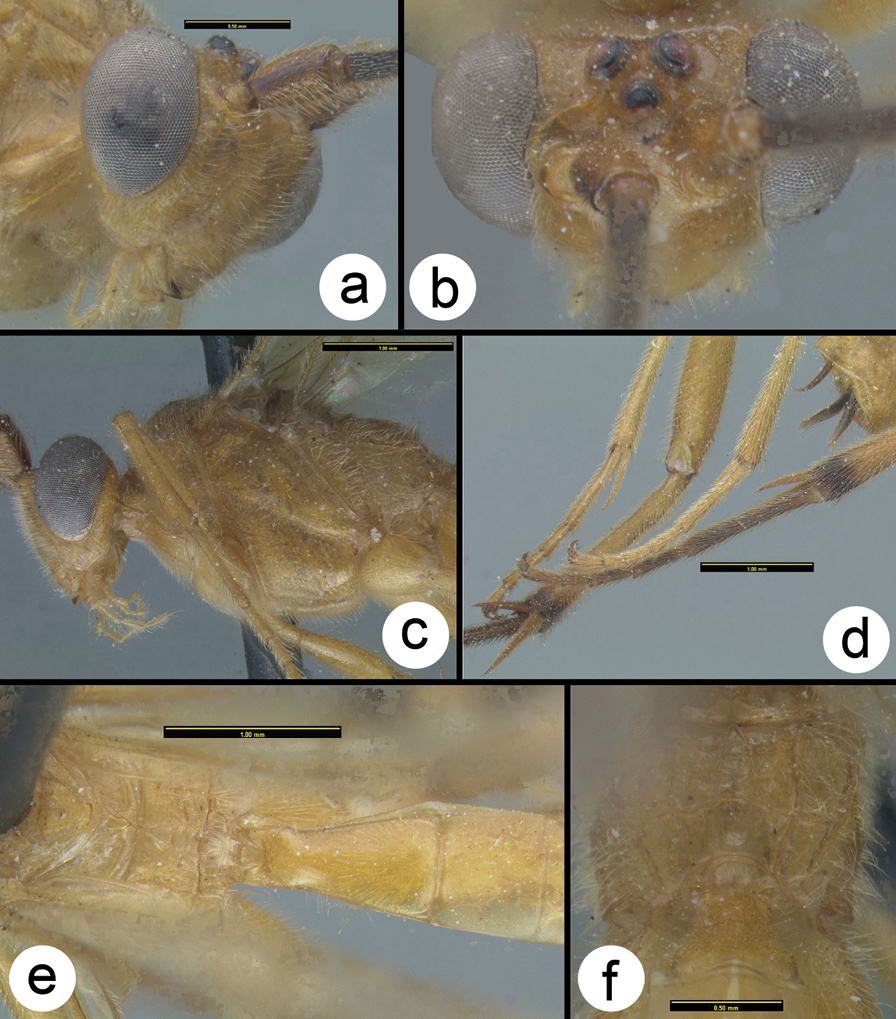
Braunsia fumipennis (Cameron) a dorsal head b lateral head c lateral mesosoma d dorsal mesothorax e dorsal propodeum f dorsal metasomal tergum 1 g dorsal metasomal terga 2 and 3.
H393: #HQ667947. H915: #HQ667946. H140: #HQ667975.
North eastern India, Myanmar, Thailand, and Vietnam. This is a wide distribution but the specimens from disparate areas vary only in minor details. Distribution map from Thailand and Myanmar can be found at http://purl.org/thaimaps/fumipennis.
Examined specimens are deposited in OUMNH, RMNH, BMNH, HIC, and QSBG.
http://species-id.net/wiki/Braunsia_smithii
Figs 13–14Similar species include Braunsia maculifera van Achterberg and Long, Braunsia pappi
Braunsia smithii (Dalla Torre) a lateral habitus b fore wing.
Braunsia smithii (Dalla Torre) a anterior head b lateral head c lateral mesosoma d dorsal mesothorax e dorsal propodeum and metasomal terga 1–3.
The type of B. smithii is from Ceram Island, Indonesia (Wallacea). Thus the geographic range of the species is wide. Despite this, examination of specimens from this wide range provides no morphological grounds on which to separate the two nominal species. As a warning to future revisers, all older specimens examined, and these include specimens from Peninsular Malaysia and Borneo, have an oily residue that causes the cuticle to darken in a variety of patterns depending on where the substance contacts the cuticle. This is especially apparent on the metasoma.
H292: #HQ667948. H906: #HQ667949.
Examined specimens are from Malaysia (Borneo and Peninsular Malaysia), Thailand, Vietnam, and Indonesia (South Moluccas). It is undoubtedly much more widespread. Distribution map can be found at http://purl.org/thaimaps/smithii.
Specimens are deposited in OUMNH, RMNH, MZPW, BMNH, HIC, UKM, and QSBG.
http://species-id.net/wiki/Braunsia_sumatrana
Figs 15–16Large specimens with unique coloration; especially noteworthy is the extent of white coloration on the first metasomal median tergite.
Braunsia sumatrana Enderlein a lateral habitus b dorsal habitus.
Braunsia sumatrana Enderlein a dorsal head b lateral head and mesosoma c dorsal head and mesosoma d wings e ovipositor and sheaths.
Indonesia (Sumatra), Malaysia (Borneo and Peninsular Malaysia). It is included here because of the likelihood that its range extends into southern Thailand. Distribution map can be found at http://purl.org/thaimaps/sumatrana.
Examined specimens are deposited in HIC, UKM, RMNH, USNM.
Key to Thai species of Camptothlipsis
| 1a | Metasomal terga almost entirely orange, and lacking a distinct white band, head entirely orange | Camptothlipsis hanoiensis van Achterberg & Long |
| 1b | Metasomal terga mostly or entirely melanic with or without a pale transverse band on median tergite 2 | 2 |
| 2a | Mesoscutum almost entirely orange | Camptothlipsis philippinensis Gupta & Bhat |
| 2b | Mesoscutum entirely melanic or almost so | 3 |
| 3a | Penultimate labial palpomere about ½ the length of the apical palpomere (Note: the second palpomere, that which is next to the basal palpomere, is present and it is the thickest palpomere; in this side of the couplet there should be two palpomeres distal to it); mid tibia with more than 8 lateral spines | Camptothlipsis nigra Gupta & Bhat |
| 3b | Penultimate labial palpomere absent or barely perceptible (Note: only one apparent palpomere distal to the thickest palpomere); mid tibia with less than 6 lateral spines | 4 |
| 4a | Posterodorsal corner of pronotum orange | Camptothlipsis sheilaeSharkey, sp. n. |
| 4b | Posterodorsal corner of pronotum melanic | Camptothlipsis annemariaeSharkey, sp. n. |
urn:lsid:zoobank.org:act:616728A7-5F48-403D-8FD9-5BE69950601B
http://species-id.net/wiki/Braunsia_annemariae
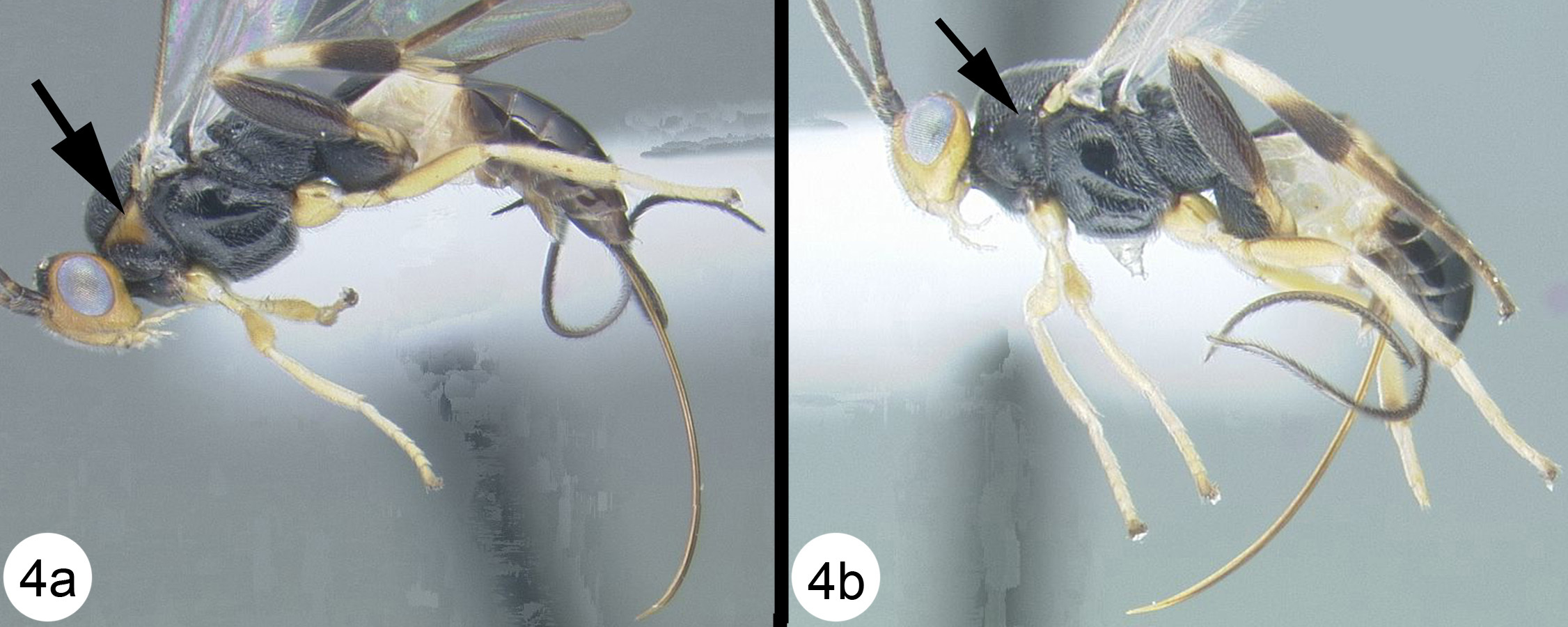
Figs 17–18Very similar to its sister species, Camptothlipsis sheilae. Similarities include median tergite 2 weakly granulate, pronotum mostly smooth posterodorsally (in the lateral corner) and 28S sequence data that differ at only 3 sites (of roughly 600 base pairs total) (Fig. 2). Camptothlipsis annemariae differs as follows: pronotum melanic orange in posterolateral corner, ovipositor distinctly shorter than body (see couplet 4 above to compare ovipositor lengths).
Holotype female.
Body length. 3.0 mm.
Head. Third labial palpomere not visible under a light microscope at 80x (uncertain whether or not a small palpomere remains), OOL 0.14; POD 0.05; IOL 0.11; 27 flagellomeres.
Mesosoma. Pronotum mostly smooth posterodorsally (in the lateral corner); sternaulus well impressed and distinctly crenulate for ¾ mesopleuron length; propodeum evenly areolate rugulose with small areolae; midleg with 5–6 spines; hind leg with 5 spines.
Metasoma. First metasomal median tergite (T1) 1.3 times longer than wide apically and distinctly granulate; T2 weakly granulate, remaining median tergites smooth.
Male. Unknown.
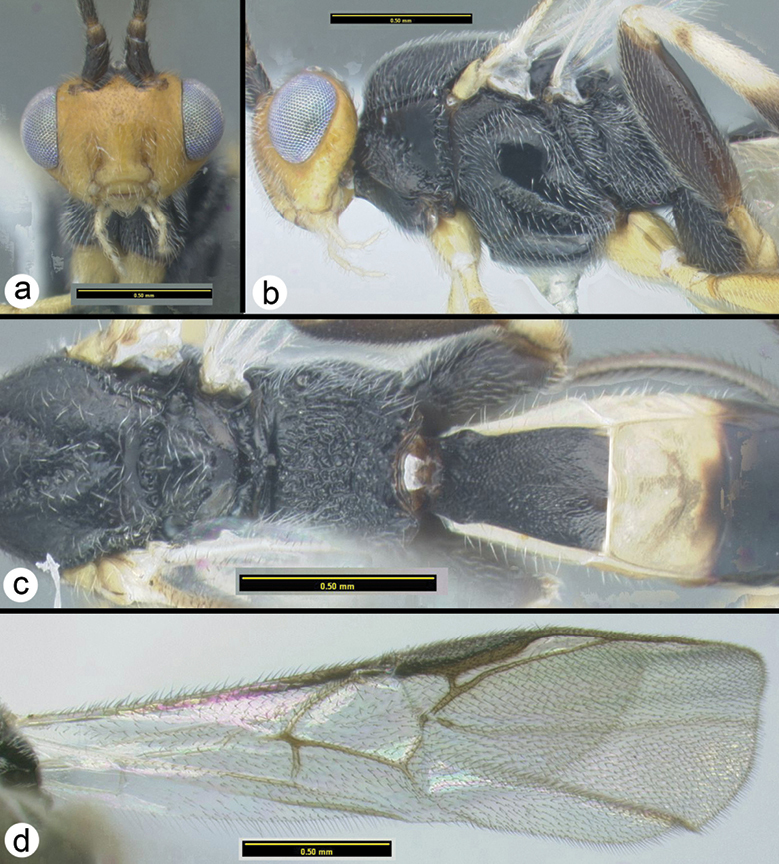
Camptothlipsis annemariae sp. n. a lateral habitus b dorsal habitus.
Camptothlipsis annemariae sp. n. a anterior head b lateral head and mesosoma c dorsal mesosoma and metasomal terga 1 and 2 d fore wing.
H394: #HQ667953
Known only from the type specimen in Thailand. Distribution map can be found at http://purl.org/thaimaps/anemariae.
Named in honor of the first author’s sister, Annemarie.
Holotype female: H394, Thailand, Petchaburi, Kaeng Krachan NP, Panernthung/km27, 12.822°N, 99.371°E, MT, 25.i-4.ii.2009, Sirichai [QSBG].
http://species-id.net/wiki/Camptothlipsis_hannoiensis
The extensive pale color of the body is not found in other Thai species.
The species is only known from Vietnam and is included here due to the possibility that it may occur in Thailand. See
http://species-id.net/wiki/Camptothlipsis_nigra
Figs 19–22NOTE: The generic name is feminine therefore the code requires that gender of the species name be changed to agree.
Quite variable in coloration; mesosoma from mostly black to extensively pale especially laterally; median tergite 2 from completely pale to mostly melanic with a pale transverse band basally. Third labial palpomere well developed, more than 9 spines on the mid tibia. Figures 19, 20 and 21 are of Thai specimens, figure 22 illustrates the holotype.
H096: #HQ667952. H433: #HQ667951. H546: #HQ667957. H1627: #HQ667955. H1635: #HQ667956.
The holotype is well described in
Body length: 3.7 - 3.8 mm. Penultimate labial palpomere well developed. OOL 0.17; POD 0.06; IOL 0.10; 26 flagellomeres. Mid tibia with 9–10 spines; hind tibia with 12–13 spines. First metasomal median tergite (T1) 1.3 times longer than wide apically and distinctly granulate; sculpture of T2 distinctly granulate, remaining median tergites smooth.
Camptothlipsis nigra Gupta and Bhat, melanic specimen a lateral habitus b wings.
Camptothlipsis nigra Gupta and Bhat, pale specimen a lateral habitus b dorsal habitus.
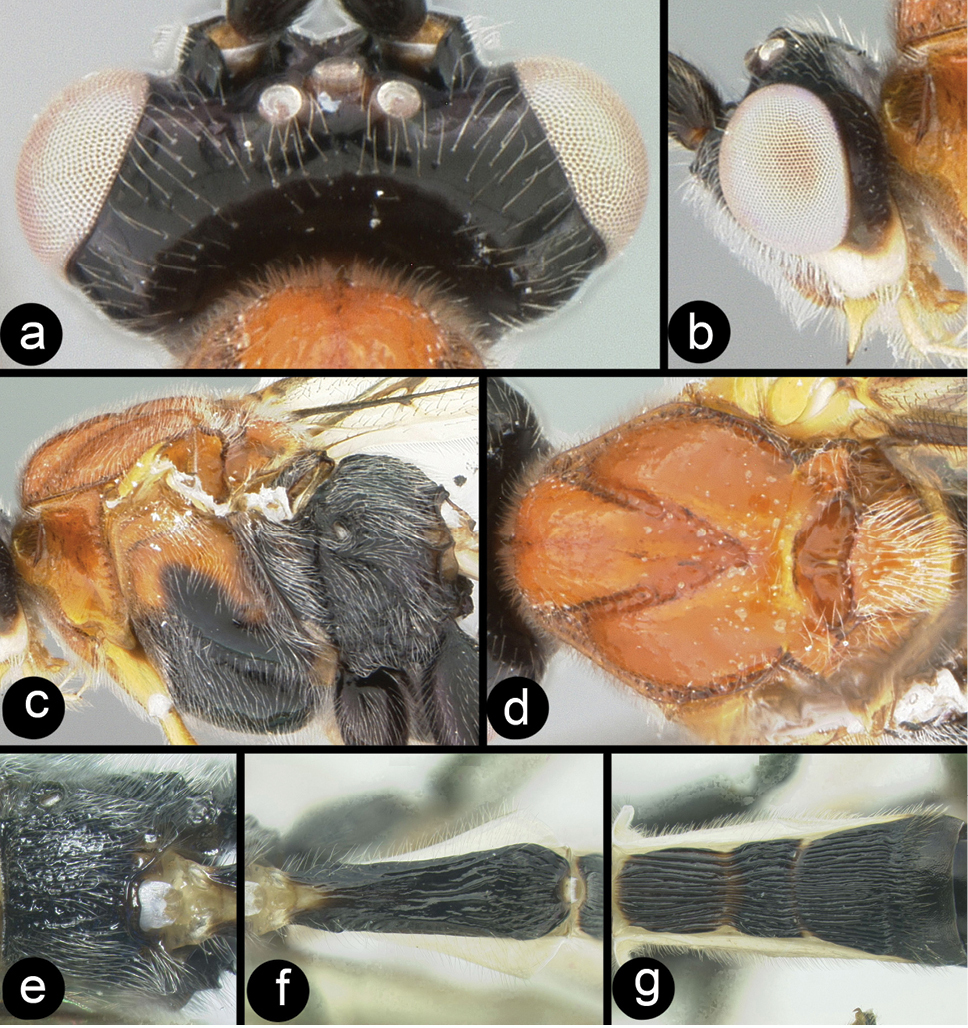
Camptothlipsis nigra Gupta and Bhat, melanic specimen a anterodorsal head b lateral head c lateral mesosoma d dorsal mesosoma e dorsal propodeum and metasomal terga 1–3.
Camptothlipsis nigra Gupta & Bhat, holotype, lateral habitus.
The holotype is from Java and other specimen records include eastern India and Thailand. Distribution map of the Thai specimens can be found at http://purl.org/thaimaps/nigra.
Specimens are deposited in the USNM (holotype, and paratypes), CNC (paratypes), other examined specimens are deposited in QSBG, HIC and RMNH.
http://species-id.net/wiki/Camptothlipsis_philippinensis
Figs 23–26The pale color of this species is enough to distinguish it from others in Thailand. The penultimate labial palpomere is well developed. Figures 22 and 23 are of the sole Thai specimen, a male. Figures 24 and 25 are of the holotype.
Camptothlipsis philippinensis Gupta & Bhat a lateral habitus b dorsal habitus.
Camptothlipsis philippinensis Gupta & Bhat a anterior head b dorsal propodeum and metasomal terga 1 and 2 c lateral head and mesosoma d dorsal head and mesothorax e fore wing f hind wing.
Camptothlipsis philippinensis Gupta & Bhat, holotype a lateral habitus b dorsal habitus.
Camptothlipsis philippinensis Gupta & Bhat, holotype a anterior head b fore wing c lateral mesosoma d dorsal mesosoma e dorsal propodeum and metasomal terga 1 and 2.
Known only from Philippines and Thailand. Distribution map of the Thai specimens can be found at http://purl.org/thaimaps/philippinensis.
The holotype, from The Philippines, is deposited in the USNM and the Thai specimen is in QSBG.
urn:lsid:zoobank.org:act:B1A13E1D-79A9-4572-89FD-4BF59E461905
http://species-id.net/wiki/Camptothlipsis_sheilae
Figs 27– 28Very similar to its sister species, Camptothlipsis annemariae. Similarities include median tergite 2 weakly granulate; pronotum mostly smooth posterodorsally (in the lateral corner) and 28S sequence data that differ at 3 sites (Fig. 2). Camptothlipsis sheilae differs as follows: pronotum yellowish orange in posterolateral corner, ovipositor about as long as body.
Holotype female.
Body length. 3.4 mm.
Head. Third labial palpomere not visible under a light microscope at 80x (uncertain if a small palpomere remains) OOL 0.15; POD 0.06; IOL 0.10; 26 flagellomeres.
Mesosoma. Pronotum mostly smooth posterodorsally (in the lateral corner); sternaulus well impressed and distinctly crenulate for ¾ mesopleuron length; propodeum evenly areolate rugulose with small areolae; mid leg with 4 spines; hind leg with 7 spines.
Metasoma. First metasomal median tergite (T1) 1.3 times longer than wide apically and distinctly granulate; T2 weakly granulate, remaining median tergites smooth.
Color. Head orange with black in ocellar triangle. Most of mesosoma black; posterolateral corner of pronotum yellowish orange. Median tergites melanic except median tergite 2 yellowish white.
Male. Unknown.
Camptothlipsis sheilae sp. n. a lateral habitus b dorsal habitus.
Camptothlipsis sheilae sp. n. a anterior head b lateral head and mesosoma c dorsal head and mesothorax d dorsal propodeum and metasomal terga 1 and 2. Arrows show positions for length and width measurements e fore wing.
H664: #HQ667954
Known only from the type specimen in Thailand. Distribution map can be found on http://purl.org/thaimaps/sheilae.
Named in honor of the first author’s sister, Sheila.
Holotype female: H664, Thailand, Kanchanaburi, Khuean Srinagarindra NP, Tha Thung-na/Chong Kraborg, 14.5°N, 98.884°E, 210m, MT, 9–16.iv.2009 Boonnam & Phumarin [QSBG].
Coccygidium
Key to Thai species of Coccygidium
| 1a | Fore wing melanic distally with a melanic spot near parastigma. Scape entirely melanic or with some weak pale infusions medially | Coccygidium phaeoscapos Sharkey, sp. n. |
| 1b | Fore wing weakly melanic distally with a basal transverse melanic band posterad parastigma (note the dark veins in this area). At least half of scape pale; scape half melanic (laterally) and half yellow (medially) | Coccygidium mastigion Sharkey, sp. n. |
| 1c | Fore wing mostly hyaline with slight infuscation distally and small melanic area around parastigma; scape mostly yellow with a thin melanic stripe laterally | Coccygidium malayensis(Bhat & Gupta) |
http://species-id.net/wiki/Coccygidium_malayensis
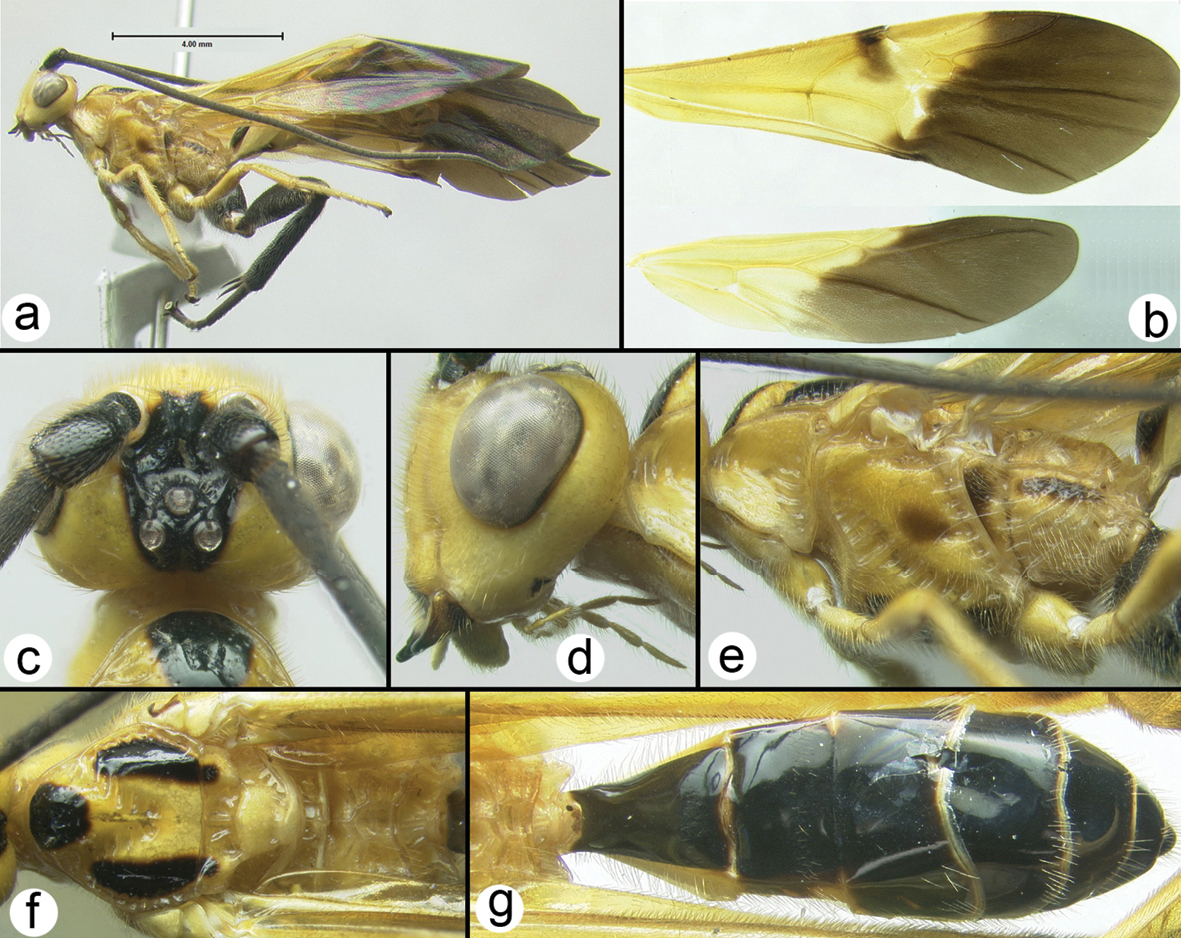
Figs 29–32Fore wing almost evenly hyaline; scape with a narrow melanic band laterally. There is a widespread undescribed species in peninsular Malaysia that is very similar to Coccygidium malayenisis. The undescribed species differs as follows: ocular- ocellar length (OOL) longer, almost twice as long as lateral ocellar diameter. Yellow coloration on stigma less than 1/3 of stigma surface.
Body length. 6.7mm
Head. OOL 0.19; POD 0.14; IOL 0.08; EH 0.85; MS 0.20; 46 flagellomeres.
Mesosoma: Mesoscutum punctate; notauli well impressed and crenulate; scutellum with apical carina and subapical transverse depression; sternaulus well impressed and crenulate, extending about ¾ length of mesopleuron; metapleuron rugose in ventral 1/3, punctate dorsally; fore tibial spur as long as basitarsus.
Metasoma. Median tergite 1 about twice as long as wide apically (length = 1.11mm, width = 0.54mm).
Color. Yellow except as follows: flagellum melanic, scape yellow except for narrow melanic stripe laterally, pedicel yellow; tip of hind tibia black, hind tarsomeres brown to black, hind tibial spurs pale brown. Fore wing almost evenly hyaline, veins mostly yellow, somewhat more melanic apicoanteriorly; stigma yellow in basal 1/3 to 1/2, vein R pale from stigma to union with RS. Hind wing hyaline with yellow veins.
Male. Eyes are somewhat smaller than those of the female. This may be typical of most nocturnal species of Coccygidium. OOL 0.22; POD 0.13; IOL 0.12; EH 0.69; MS 0.29; 45 flagellomeres.
Coccygidium malayensisholotype a lateral habitus b wings.
Coccygidium malayensisholotype a anterolateral head b dorsal head c lateral head and mesosoma d legs e dorsal propodeum and metasomal terga 1 and 2 f dorsal propodeum.
Coccygidium malayensisa lateral habitus b wings.
Coccygidium malayensis a dorsal head b lateral head and prothorax c dorsal mesoscutum d dorsal propodeum e lateral mesosoma f dorsal metasomal terga 1 and 2.
H071: #HQ667960. H075: #HQ667959. H086: #HQ667961. H971: # HQ667958
Thailand and Peninsular Malaysia. Distribution map can be found at http://purl.org/thaimaps/malayensis.
Holotype female. Malaysia, Kuala Lumpur, Selangor, 1950, AEI. Identified specimens are deposited in HIC, AEI, RMNH, QSBG, and UKM.
urn:lsid:zoobank.org:act:064BB16F-9390-4E81-8EBB-ACB8ADBF573F
http://species-id.net/wiki/Coccygidium_mastigion
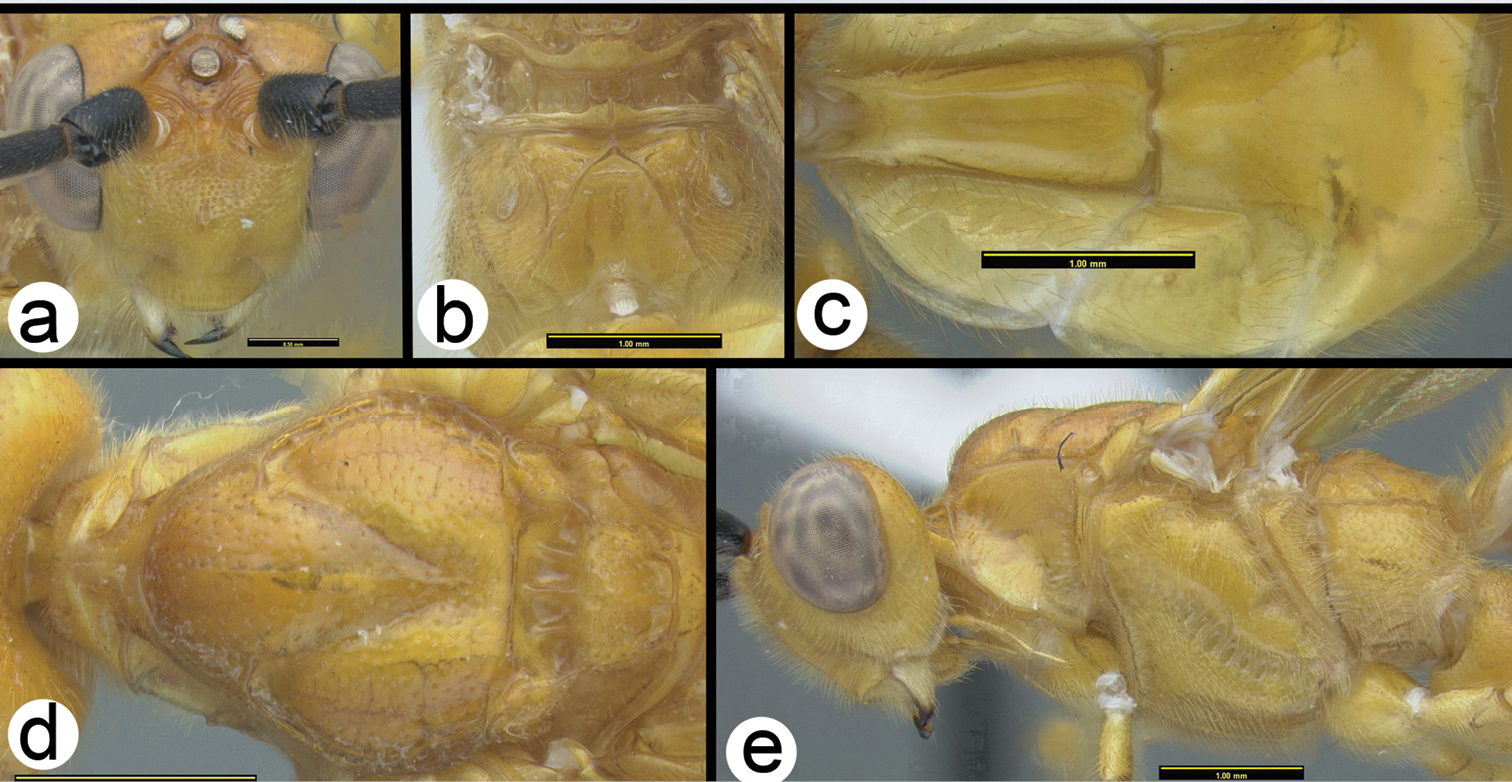
Figs 33–34Fore wing weakly melanic distally with a basal transverse melanic band posterad parastigma. Scape half melanic (laterally) and half yellow (medially).
Holotype female.
Body length. 6.12 mm
Head. OOL 0.13; POD 0.15; IOL 0.09; EH 0.88; MS 0.16; 38 (38–40) flagellomeres.
Mesosoma. Mesoscutum punctate; notauli well impressed and crenulate; scutellum with apical carina and subapical transverse depression; sternaulus well impressed and crenulate, extending about ¾ length of mesopleuron; metapleuron rugose in ventral 1/3, punctate dorsally; fore tibial spur as long as basitarsus.
Metasoma. Median tergite 1 about twice as long as wide apically (length = 0.94mm, width = 0.48mm).
Color. Yellow, except as follows: flagellum melanic, scape melanic in lateral half, yellow in medial half, pedicel melanic; tip of hind tibia black, hind tarsomeres mostly black but each paler basally, hind tibial spurs yellow to pale brown. Fore wing weakly infumate in distal half and in a longitudinal stripe basad stigma, veins yellow basally, melanic where the membrane is weakly melanic; stigma yellow in basal half, vein R pale from stigma to union with RS. Hind wing hyaline with yellow veins.
Male. Eyes smaller than those of female. OOL 0.17; POD 0.15; IOL 0.11; EH 0.79; MS 0.23; 40 flagellomeres.
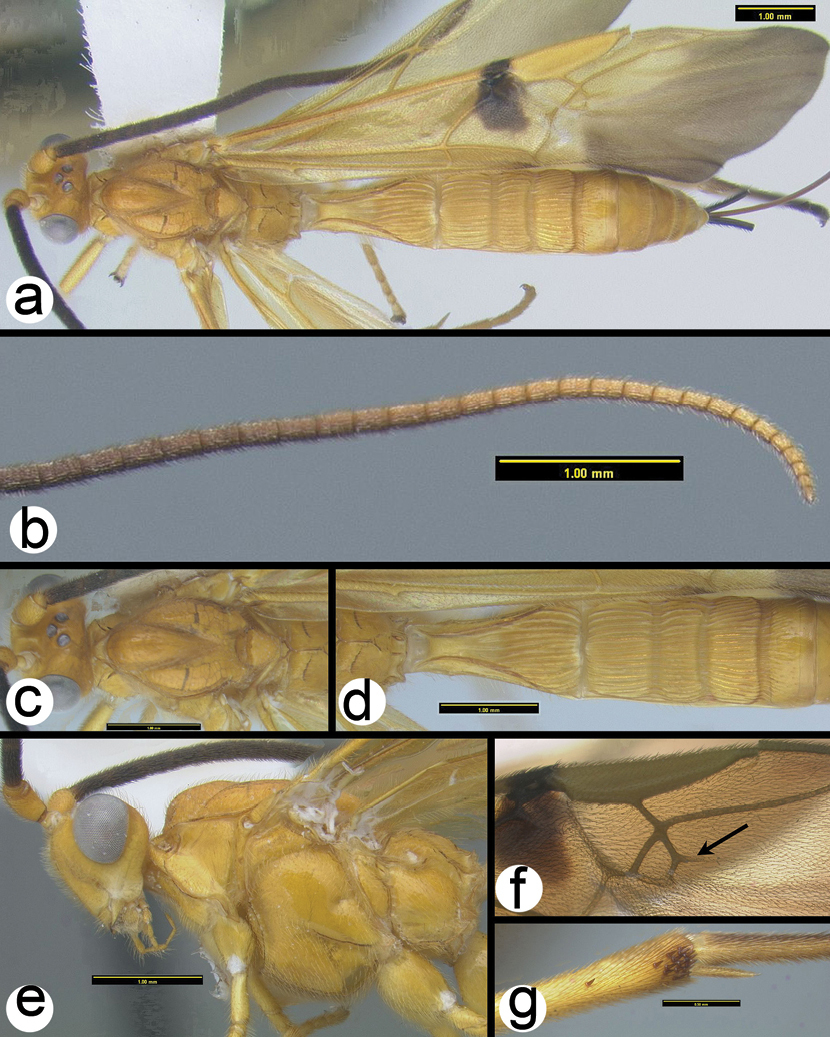
Coccygidium mastigon a lateral habitus b wings.
Coccygidium mastigon a lateral head and mesosoma b dorsal head and mesoscutum c dorsal propodeum and metasomal tergum 1.
H102: #HQ667977. H680: #HQ667962.
Known only from Thailand. Distribution map can be found at http://purl.org/thaimaps/mastigion.
Mastigion is the diminutive form of the Greek word mastix; it is a reference to the short flagellum of this species.
Holotype female: H102, Thailand, Chaiyaphum, Tat Tone NP, lawn near Sab Somboon forest unit, 16.013°N, 101.975°E, 648m, MT, 26.xi-3.xii.2006, Tawit Jaruphan [QSBG], Paratype male: H680, Thailand, Chaiyaphum, Tat Tone NP, 15.9586°N, 101.9073°E, MT, 12–19.iv.2007, Tawit Jaruphan & Orawan Budsawong [HIC].
urn:lsid:zoobank.org:act:C35E0315-D198-46D3-9511-205DB307F497
http://species-id.net/wiki/Coccygidium_phaeoscapos
Figs 35–36Fore wing melanic distally with a melanic spot near parastigma. Scape entirely melanic or with some weak pale infusions medially.
Holotype female.
Body length. 7.1 mm
Head. OOL 0.17; POD 0.17; IOL 0.12; EH 1.04; MS 0.18; 42 flagellomeres.
Mesosoma: Mesoscutum punctate; notauli well impressed and crenulate; scutellum with apical carina and subapical transverse depression; sternaulus well impressed and crenulate, extending about ¾ length of mesopleuron; metapleuron rugose in ventral 1/3, punctate dorsally; fore tibial spur distinctly longer than basitarsus.
Metasoma. Median tergite 1 about twice as long as wide apically (length = 1.11mm, width = 0.59 mm).
Color. Yellow except as follows: flagellum melanic, scape entirely melanic or rarely with some weak pale infusions medially, pedicel melanic; tip of hind tibia varying from black to brown, hind tarsomeres varying from dark yellow to black, hind tibial spurs yellow to pale brown. Fore wing infumate in distal half and with a dark spot near parastigma, veins yellow basally, melanic distally; stigma yellow in basal half, vein R pale from stigma to union with RS. Hind wing yellowish hyaline basally and distinctly melanic distally.
Male. Unknown.
Coccygidium phaeoscapos a lateral habitus b wings.
Coccygidium phaeoscapos a lateral head and mesosoma b dorsal head and thorax c dorsal propodeum and metasomal terga 1 and 2.
H265: #HQ667965. H266: #HQ667964. H267: #HQ667967. H342: #HQ667966. H560: #HQ667968. H671: #HQ667963.
Known only from Thailand. Distribution map can be found at http://purl.org/thaimaps/phaeoscapos.
From the Greek phaios meaning dusky or brown, and scapos; this is a reference to the dark scape which distinguishes this species.
Holotype female: H267, Thailand, Ubon Ratchathani, Pha Taem NP, Phu Krajeaw foothill 15.667°N, 105.508°E 246m MT 2–9.vi.2007 Tongcam & Banlu [QSBG].
Paratypes ♀: Thailand: H671, Chaiyaphum, Pha Hin Ngam NP, deciduous forest/ Tepa Waterfall, 15.649°N, 101.418°E, 614m, MT, 19–25.iv.2007, Katae Sa-nog & Buakaw Adnafai [QSBG]; H265, Chaiyaphum, Pa Hin Ngam NP, deciduous forest, 15.666°N, 101.453°E, 357m, MT, 13–19.vi.2007, Katae Sa-nog & Buakaw Adnafai[ HIC]; H342, Loei, Phu Kradueng NP, Mixed deciduous/S Na Noy office 16.817°N, 101.794°E, 276m, MT, 14–21.v.2008, Thonghuay Phatai; H266, Sakon Nakhon, Phu Phan NP16.81°N, 103.892°E, 512m, MT, 16–22.vi.2007, Winlon Kongnara [QSBG]; H631, Tat Tone NP, 16°0.79N, 101°58.472E, 648m, MT, 12–19.v.2007, Jaruphan & Budsawong [QSBG]; H553 & H560, Chaiyaphum, Tat Tone NP, Dipterocarp forest at Sapsomboon substation 16.018°N, 101.977°E, 674m, MT, 19–26.v.2007, Tawit Jaruphan & Orawan Budsawong [QSBG, HIC]; H306, Chaiyaphum, Tat Tone NP, Dipterocarp forest at Sapsomboon substation, 16.043°N, 101.977°E, 675m MT, 5–12.v.2007, Tawit Jaruphan & Orawan Budsawong [QSBG]; H395 & H398, Chaiyaphum, Tat Tone NP, Dipterocarp forest at Sapsomboon substation, 16.043°N, 101.977°E, 675m, MT, 26.v.-2.vi.2007, Tawit Jaruphan & Orawan Budsawong [QSBG, HIC].
Key to Thai Species of Cremnops
| 1a | Metasoma and hind leg mostly or entirely pale | 2 |
| 1b | Metasoma and hind leg mostly or entirely melanic | Cremnops fuscipennis Brullé |
| 2a | Fore wing variable but never as in 2b and usually with a yellow stigma area and two or three pale bands | Cremnops desertor (Linnaeus) |
| 2b | Fore wing mostly deep yellow with the distal edge infumate and a small infumate patch below the parastigma (The latter may be variable.) | Cremnops mekongensis Turner |
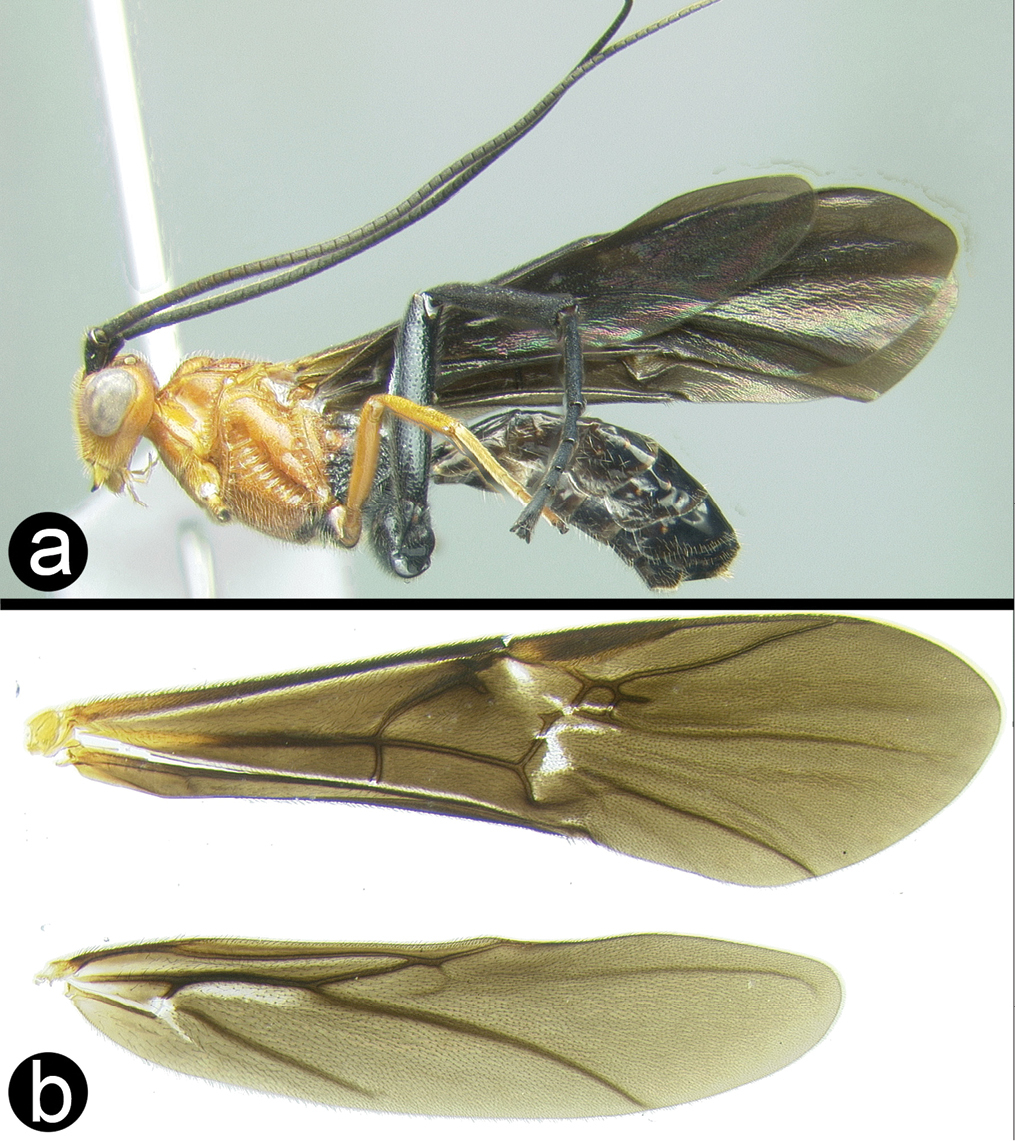
http://species-id.net/wiki/Cremnops_desertor
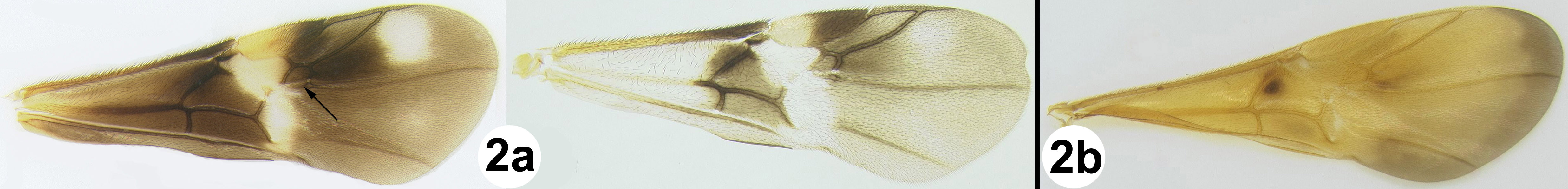
Fig. 37This is a highly variable and widespread species. The fore wing varies from completely infuscate to mostly hyaline with dark and yellow bands. The images in the key (above) show a good range of variation. The combination of characters given in the key is sufficient to identify Thai specimens.
Cremnops desertor a lateral habitus b fore wing.
Contrary to
H098: #JF506256. H8099: #JN019810. H294: #JN019811
Widespread over temperate and tropical Eurasia and, recently, accidentally introduced to eastern North America. Distribution map of Thai and Peninsular Malaysia specimens can be found at http://purl.org/thaimaps/desertor.
Examined specimens are in the following collections: BMNH, HIC, QSBG, CNC and UKM.
http://species-id.net/wiki/Cremnops_fuscipennis
Fig. 38Fore wing evenly and darkly infuscate, with or without a small clear patch posterior to the stigma; hind leg and metasoma black; head yellow with black vertex, mesosoma yellow except metathorax and propodeum which vary from yellow to black; notauli smooth and weakly impressed, especially anteriorly.
Cremnops fuscipennis a lateral habitus b dorsal head and thorax.
Very similar to Cremnops collaris
Examined specimens are from Java and Peninsular Malaysia. Literature records are only from Java. Although the species has not been recorded in Thailand it is included here due to the likelihood that its range extends into southern Thailand. Distribution map of Peninsular Malaysia specimens can be found at http://purl.org/thaimaps/fuscipennis.
Examined specimens are in the following collections: BMNH, HIC, and UKM.
http://species-id.net/wiki/Cremnops_mekongensis
Figs 39–40Easily distinguished from other species by body and/or fore wing color. This is only the second specimen of the species collected, the other being the holotype.
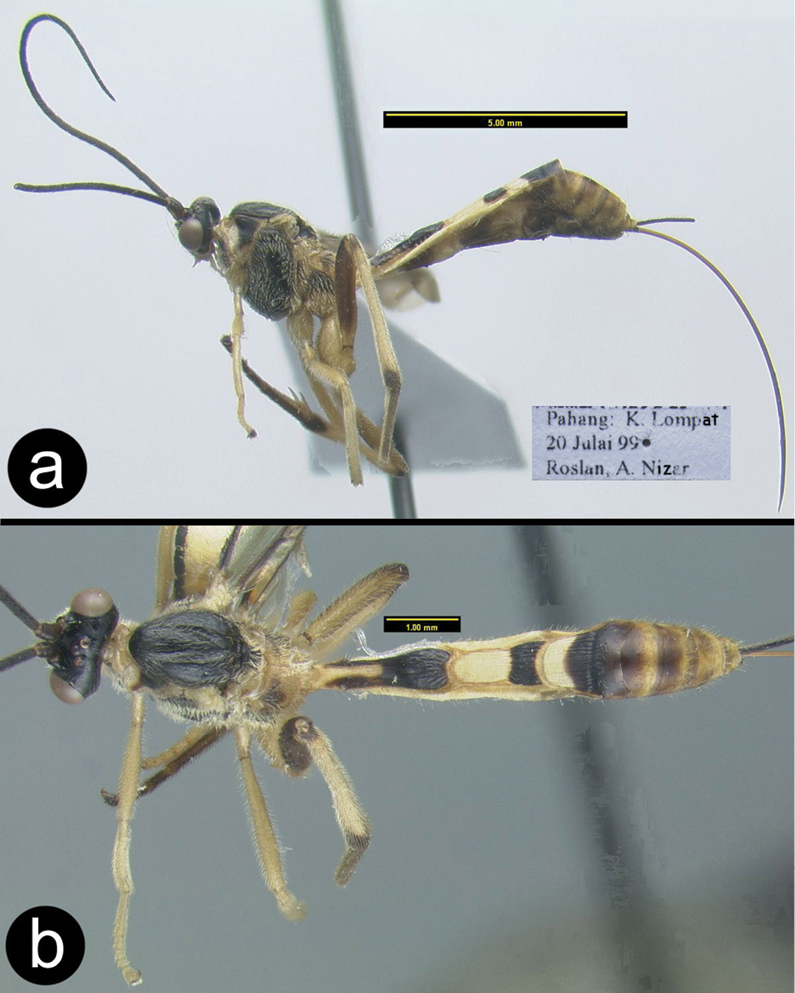
Cremnops mekongennsis a lateral habitus, inset showing ovipositor length b wings.
Cremnops mekongennsis a anterior head b lateral head and mesosoma c dorsal thorax d dorsal propodeum and metasomal tergum 1.
H470: #HQ667976
Thailand and Laos. Distribution map of the sole Thai specimen deposited in HIC can be found at http://purl.org/thaimaps/mekongensis.
urn:lsid:zoobank.org:act:A118E588-FD32-49E4-AC32-A9D47FCB2CFB
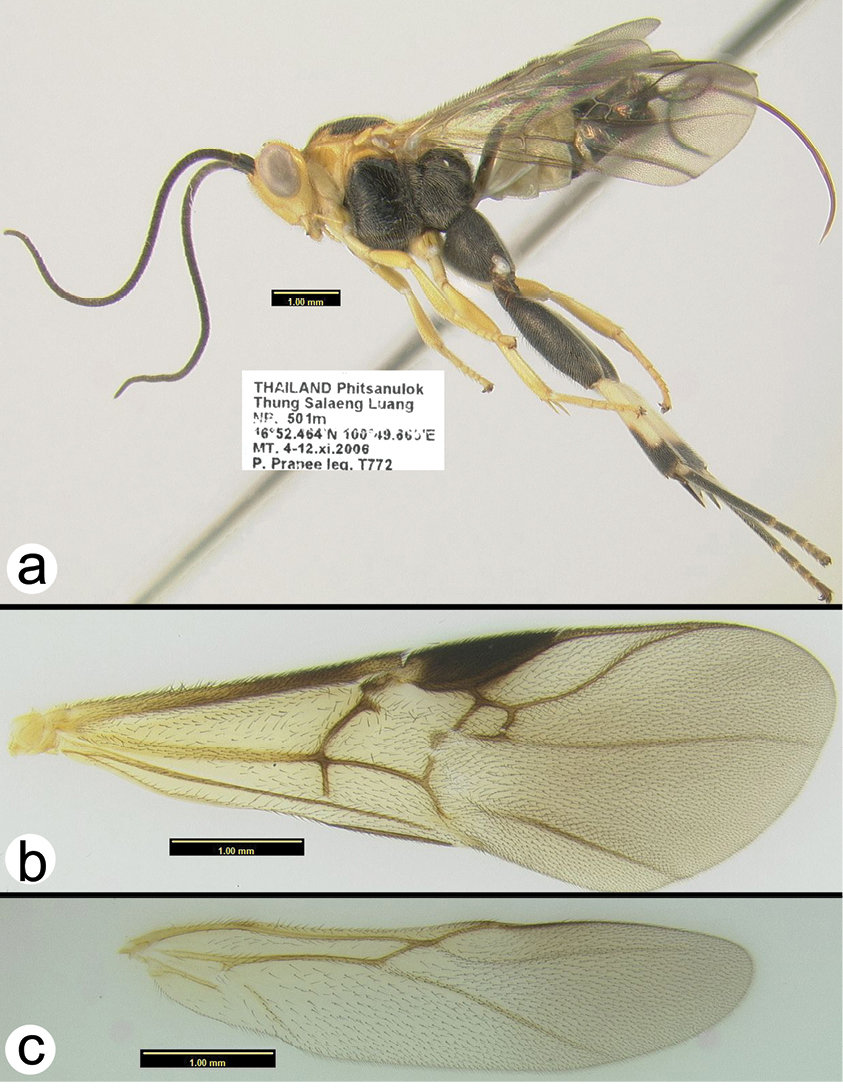
http://species-id.net/wiki/Cremnoptoides_yui
Figs 41–42This is the only species of Cremnoptoides that is not almost entirely black. The other two species, Cremnoptoides furcatus and Cremnoptoides pappi, do not have the extensive yellow coloration present in Cremnoptoides yui. For descriptions of the other two species see
Holotype female.
Length. 7.0 mm.
Head. Antenna with 40 flagellomeres; head slightly elongate; frons with sharp lateral carinae extending to lateral ocelli.
Mesosoma. Sternaulus weakly impressed with elongate transverse, shallow carinae extending the length of the mesopleuron; metapleuron aciculate to rugose and moderately setose; propodeum areolate with a spindle-shaped medial cell; propodeum moderately setose except medial cell; hind trochantellus with longitudinal carina, medial and lateral hind claws both with a right-angled or slightly acute basal lobe, hind femur aciculate; mid tibia with one peg; hind tibia with 3 pegs; last abscissa of RS of fore wing sinuate.
Metasoma. Metasomal tergum 1 with a smooth transverse groove; border between tergum 2 and 3 marked by a smooth transverse groove; ovipositor slightly shorter than body length.
Male. Essentially as in the holotype.
Cremnoptoides yui a lateral habitus b fore wing c hind wing.
Cremnoptoides yui a anterodorsal head b dorsal propodeum c lateral head and mesosoma d dorsal head and thorax e dorsal metasomal terga 1–4.
The holotype is representative of darker specimens. Other specimens may have more extensive yellow color on the metasomal tergites and the flagellum may be pale brown, almost dark yellow.
Known only from the above localities in Thailand. Distribution map can be found at http://purl.org/thaimaps/yui.
Named in honor of Dr. Dicky Yu, for his immense contributions to hymenopterology.
Holotypefemale: H039, Thailand, Phitsanulok, Thung Salaeng Luang NP, Mixed deciduous forest (Gang Sopa waterfall), Malaise trap, 4–12.xi.2006, Pongpitak Pranee, [QSBG].
Paratypes: Thailand: 1♀, H1182, Chiang Mai, Pa Huay Tong Moo 8 Tambon Bo Luang, 1–20.ix.1997, S. Sonthichai, [HIC]; 2♀♀, H1183 & H1184, Loei, Phu Kradueng NP, Huay Lao Kao, Malaise trap, 9–16.viii.2006, Sutin Khonglasae, [HIC, QSBG]; 2♀♀, H632 & H644, Phetchabun, Nam Nao NP, Check point, Malaise trap, 5–12.v.2007, Noopean Hongyothi, [HIC]; 1♀, H681, Suphanburi, Pu Toei NP, Phu Toei hill top/road, Malaise trap, 1–8.viii.2008, Saunbua. L. , [QSBG]; 1♀, H1185, Ubon Ratchathani, Pha Taem NP, Don Huay Can, Malaise trap, 9–15.vi.2007, Tongcam & Banlu, [QSBG]; 1♀, H1181, Ubon Ratchathani, Pha Taem NP, Huay Sa Nhom plateau, Malaise trap, 11–18.xi.2006, Sorawit and Thongdee, [QSBG]; 1♂, H1186, Ubon Ratchathani, Pha Taem NP, Kua nang nee, Malaise trap, 25.viii-2.ix.2006, Bunlu Subsiri, [HIC]; 1♀, H766, Ubon Ratchathani, Pha Taem NP, Phu Krajeaw foothill, Malaise trap, 9–15.vi.2007, Tongcam & Banlu, [HIC]; 1♀, H001, Chaiyaphum Tat Tone NP, Malaise Trap, 12–19.x.2006, Tawit Jaruphan, [HIC].
Key to Thai Species of Disophrys
| 1a | Mesoscutum yellow with a black spot on each lobe; notauli with wide crenulations | Disophrys strigata Enderlein |
| 1b | Mesoscutum reddish orange; notauli with wide crenulations | Disophrys erythrocephala Cameron |
| 1c | Mesoscutum yellow; notauli without wide crenulations | 2 |
| 2a | Blunt tubercles between antennae | Disophrys rhinoides van Achterberg & Long |
| 2b | Sharp carinae between antennae | Disophrys subfaciata (Brullé) |
http://species-id.net/wiki/Dispophrys_erythrocephala
Figs 43–44The color of this species is unique to Disophrys in the region.
Disophrys erythrocephala a lateral habitus b wings.
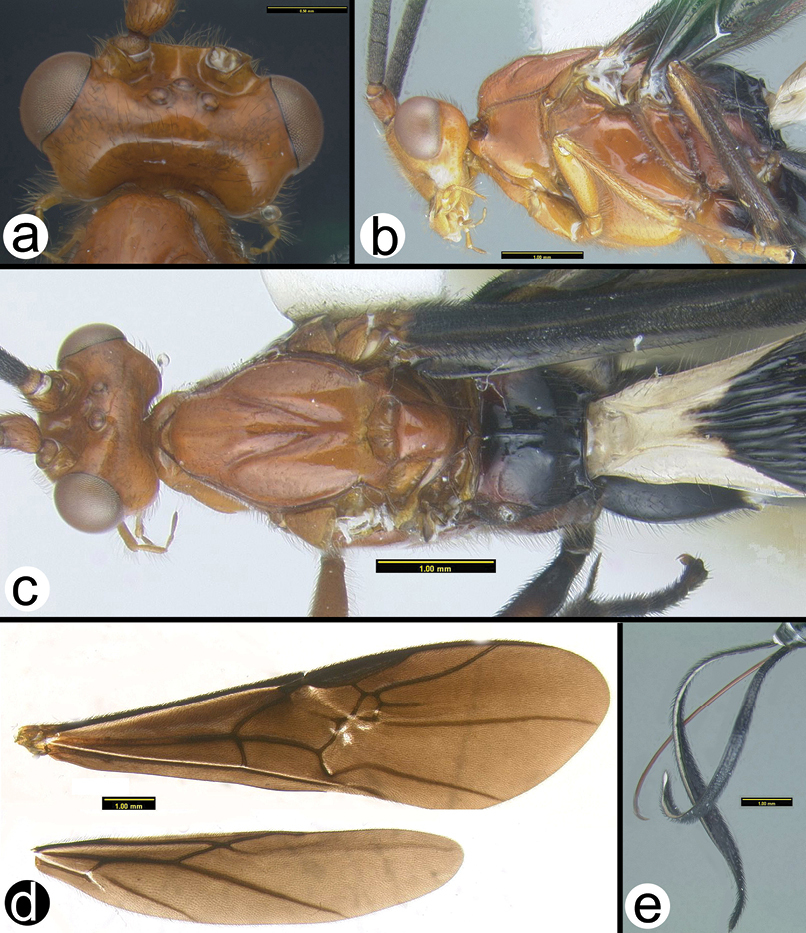
Disophrys erythrocephala a dorsolateral head b anterior head c lateral head and prothorax d dorsal mesothorax e dorsal propodeum f lateral pro- and mesothorax g dorsal metasoma.
H090: #HQ667971
Thailand, Vietnam, China, Taiwan, India, Sri Lanka, Peninsular Malaysia and Indonesia (Sumatra, Krakatau, Kangean Islands) (
Identified specimens are deposited in HIC and QSBG.
http://species-id.net/wiki/Disophrys_rhinoides
Figs 45–46The rounded protuberances between the antennae are unique.
Disophrys rhinoides a lateral habitus b wings.
Disophrys rhinoides a lateral head b anterodorsal head showing closeup of interantennal region c anterodorsal head d lateral head and mesosoma e dorsal mesosoma f dorsal propodeum and metasoma.
H007: #HQ667970
Vietnam and Thailand. Distribution map of the Thai specimens can be found on http://purl.org/thaimaps/rhinoides.
Identified specimens are deposited in HIC and QSBG.
http://species-id.net/wiki/Disophrys_strigata
Fig. 47The general color pattern and the wide crenulae on the notauli are sufficient to distinguish this species.
Disophrys strigata a lateral habitus b wings c dorsal head d lateral head e lateral mesosoma f dorsal mesosoma g dorsal propodeum and metasoma.
This species is very similar to Disophrys insignis Roman, 1913 from the Philippines but differs in that the latter has much shallower notauli and the lateral carinae of the frons are much higher than those of Disophrys macilifera. They are undoubtedly closely related in that the pattern of the propodeal carinae is unlike those of other Disophrys. Normally the medial cell of the propodeum is greatly expanded anteriorly and laterally, however in these two species it is in the form of a small pentagon. The Thai specimens are somewhat darker than those of Vietnam.
H006: # HQ667969
Indonesia, Vietnam, Thailand. Distribution map of the Thai specimens can be found at http://purl.org/thaimaps/strigata.
Identified specimens are deposited in HIC and QSBG.
http://species-id.net/wiki/Disophrys_subfaciata
Figs 48–49The color patterns, the relatively smooth notauli, and the sharp ridges between the antennae are sufficient to distinguish this species.
Disophrys subfasciata a lateral habitus b wings.
Disophrys subfasciata a anterodorsal head b dorsal metasoma and propodeum c dorsal metasomal terga 1–3 d dorsal thorax e lateral head and mesosoma.
H1862: #JF506257
Thailand, India and Vietnam. Distribution map of the Thai specimens can be found at http://purl.org/thaimaps/sybfaciata.
Besides the types, identified specimens are in HIC and QSBG.
http://species-id.net/wiki/Earinus_aurantius
Fig. 50This species is very similar to Earinus longensis
Earinus aurantius a lateral habitus b fore wing.
The Thai specimens differ from those described from Vietnam in that the former have less orange brown color on the mesosoma. In the Thai specimens this color is restricted to the apex of the scutellum.
H041: #HQ667972
Thailand and Vietnam. Distribution map of the Thai specimens which are deposited in HIC and QSBG can be found at http://purl.org/thaimaps/aurantius.
http://species-id.net/wiki/Gyrochus_yunnanensis
Fig. 51The light color of the metasoma combined with the color pattern of the wings is sufficient to distinguish this species. Gyrochus nigripennis
Gyrochus helvus
Gyrochus yunnanensis a lateral habitus b wings.
The five nominal species of Gyrochus differ almost exclusively in color pattern, particularly in the wings.
H543: # HQ667973
China (Yunnan), Vietnam, Thailand. Distribution map of the Thai specimens which are deposited in HIC and QSBG can be found at http://purl.org/thaimaps/yunnanensis.
Key to species of Thai Lytopylus
http://species-id.net/wiki/Lytophylus_ebulus
Fig. 52The orange pro- and mesothorax combined with milky white mid and hind basitarsomeres distinguish this species.
Figure 52. Lytopylus ebulus a lateral habitus b fore wing.
Here we synonymize Agathis burmensis Bhat and Gupta (1997). It differs from the holotype of Lytopylus ebulus only in minor color variation and the degree of sculpture on metasomal tergum three, which is somewhat reduced in Agathis burmensis.
Examined specimens are from India, Taiwan, Burma, and Thailand. The pale specimen figured in van Achterberg and Long (2010, Fig. 217) identified as Lytopylus romani appears to be a representative of this species. Distribution map of the Thai specimens which are deposited in QSBG can be found on http://purl.org/thaimaps/ebulus.
http://species-id.net/wiki/Lytopylus_romani
Fig. 53The melanic mesothorax combined with melanic mid and hind basitarsomeres distinguish this species in this geographic area.
Lytopylus romani a lateral habitus b fore wing.
Lytopylus romani may be an uncommonly widespread and variable species or it may represent a complex of species. We suspect that the latter is more likely. The length of the ovipositor varies considerably within the “species” with some specimens from Far East Russia possessing particularly long ovipositors. A molecular approach may be necessary to resolve species limits.
Taiwan, India, Japan, Korea, Russia, Far East Russia, Thailand. Distribution map of the Thai specimens which are deposited in QSBG can be found at http://purl.org/thaimaps/romani.
http://species-id.net/wiki/Troticus_alloflavus
Fig. 54There is one other Oriental species. Troticus giganteus
Troticus alloflavus a lateral habitus b fore wing.
Thailand and Vietnam. Distribution map of the sole Thai specimen can be found at http://purl.org/thaimaps/alloflavus.
We thank all of the staff at Queen Sirikit Botanic Garden in Chaing Mai for sorting the many hundreds of samples and for the Thai park staff for running Malaise traps and other collection devices. A special thanks to Chaweewan Hutacharern for managing the Thai end of the TIGER project. Also many thanks to the curators around the world (London, Oxford, Warsaw, Leiden, Gainesville, Budapest, Beijing, Paris, Stockholm, Washington) for lending specimens and hosting MJS on visits. Special thanks to Kees van Achterberg for lending specimens and types from Vietnam. Funding was provided by NSF grants DEB-0542864 and EF-0337220.