(C) 2011 David R. Smith. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Nine species in five genera of Nematinae are recorded for the first time from Thailand: Trichiocampus pruni Takeuchi, Dineura sharkeyi, sp. n., Moricella rueaensis, sp. n., Nematus soidaoi, sp. n., Pristiphora chalybeata Benson, Pristiphora ettera, sp. n., Pristiphora inthanoni, sp. n., Pristiphora annetna, sp. n., and Pristiphora phahompoki, sp. n. A key is given for the genera and species of Thailand. New records and description of the male are given for Pristiphora borneensis Forsius from Sabah, Malaysia, and a new record is given for Pristiphora sinensis Wong from China.
sawflies, Symphyta, southeastern Asia
Nematinae are the dominant sawflies in the arctic and subarctic regions of the world. Numbers of species drop sharply toward the south, and very few occur in tropical regions. In the Western Hemisphere, only a few extend southwards into Mexico and only six species, all Pristiphora Latreille, are native to southern Mexico, Central, and South America (
Specimens are deposited in the Queen Sirikit Botanical Garden Entomological Collection, Chiang Mai, Thailand (QSBG); the National Museum of Natural History, Smithsonian Institution, Washington, DC, USA (USNM); and the Bernice P. Bishop Museum, Honolulu, HI, USA (BPBM).
Images for plates were acquired through an EntoVision micro-imaging system. This system included a Leica M16 or Leica DRMB compound microscope with a JVC KY-75U 3-CCD digital video camera or a GT-Vision Lw11057C digital camera attached that fed image data to a notebook or desktop computer. The program Cartograph 5.6.0 was then used to merge an image series (typically representing 30 focal planes) into a single in-focus image. Lighting was achieved using techniques summarized in
Nine species in five genera were collected during the Thailand Biodiversity Survey, one species each of Trichiocampus Hartig, Dineura Dahlbom, Moricella Rohwer, and Nematus Panzer, and five species of Pristiphora.
Key to Genera and Species of Thailand| 1 | Forewing with base of vein 2A&3A present, joining to 1A to form a small basal anal cell (Fig. 1, left line); vein M joins Sc+R near Rs+M (Fig. 1, right line) | Trichiocampus pruni Takeuchi |
| – | Forewing lacking basal anal cell, 2A&3A straight; M joins Sc+R widely separated from Rs+M (as in Fig. 9, left line) | 2 |
| 2 | Forewing with vein 2r present (Figs 4, 9); left mandible in lateral view gradually tapering from base to apex | 3 |
| – | Forewing with vein 2r absent (as in Fig. 17); left mandible in lateral view with base bulbous and apical portion long, thin, and bladelike | 4 |
| 3 | Tarsal claws simple | Dineura sharkeyi sp. n. |
| – | Tarsal claws with long inner tooth, longer and stouter than outer tooth | Moricella rueaensis sp. n. |
| 4 | Clypeus emarginated (Fig. 12); tarsal claws with long inner tooth; female sheath from above narrowing to acute apex, without scopae | Nematus soidaoi sp. n. |
| – | Clypeus truncate; tarsal claws with small or long inner tooth; female sheath broad at apex, with scopae; Pristiphora | 5 |
| 5 | Female | 6 |
| – | Male (Pristiphora inthanoni and Pristiphora phahompoki unknown) | 10 |
| 6 | Third abdominal segment and underside of fourth segment white; body with bluish metallic sheen [inner tooth of tarsal claws about as long as outer tooth; hind tibial spurs half length of hind basitarsomere; hind basitarsomere equal to length of 3 following tarsomeres] (not seen, from |
Pristiphora chalybeata Benson |
| – | Abdomen black or mostly black above and white below; body without metallic sheen | 7 |
| 7 | Venter of abdomen white; legs white (at most narrow apical ring on hind tibia) (Figs 14–16); 4 cubital cells in forewing; tarsal claws with long inner tooth, equal to or nearly length of outer tooth | 8 |
| – | Venter of abdomen black; legs with more black, apical third or half of hind tibiae black, femora mostly black (Figs 17, 19); 3 cubital cells in forewing; tarsal claws with small inner tooth, about half length of outer tooth | 9 |
| 8 | Pronotum white (Fig. 14); clypeus and supraclypeal area black; serrulae of lancet shallow, flat; serrulae with anterior subapical spurette (Fig. 21) | Pristiphora ettera sp. n. |
| – | Pronotum mostly black (Fig. 15); clypeus and supraclypeal area brown; basal serrulae of lancet broad, rounded, apical serrulae flat; serrulae without anterior subapical spurette (Fig. 22) | Pristiphora inthanoni sp. n. |
| 9 | Antennal length 2.3× head width (Fig. 20); tegula black; apical 1/3 hind tibia and apical 4 tarsomeres black, basitarsomere white; forewing with intercostal crossvein; serrulae of lancet serrate, annuli with stout spines nearly as long as segment width (Fig. 24) | Pristiphora phahompoki sp. n. |
| – | Antennal length 3.7× head width (Fig. 17); tegula white; apical half of hind tibia and hind tarsus black; forewing without intercostal crossvein; serrulae of lancet deeper, more rounded at apices, annuli with short, fine hairs, length much less than segment width (Fig. 23) | Pristiphora annetna sp. n. |
| 10 | Black; penis valve with perpendicular spine across width (Fig. 29) | Pristiphora chalybeata Benson |
| – | Parts of pronotum, tegula, and legs white; penis valve with short, vertical valvispina (Figs 31, 33) | 11 |
| 11 | Antenna long, 3.7× head width; penis valve broad, rounded on dorsal and ventral margins (Fig. 31) | Pristiphora annetna sp. n. |
| – | Antenna short, about 2.2 X head width; penis valve slender, dorsal and ventral margins straight (Fig. 33) | Pristiphora ettera, sp. n. |
http://species-id.net/wiki/Trichiocampus_pruni
Figs 1–3Female. Length, 6.0 mm. Entirely black; wings uniformly infuscated. Antennal length 3.0× head width; 3rd antennomere 0.7× length of 4th antennomere. Malar space 2.0× diameter of front ocellus. Lower interocular distance 1.4× eye height. Tarsal claw with long inner tooth, almost equal in length and width to outer tooth; with very small, indistinct basal lobe. Fore and hind basitarsomere subequal in length to following 3 tarsomeres combined. Sheath from above broad at base, evenly tapering to acute apex; in lateral view rounded with long, curved hairs (Fig. 2). Lancet (Fig. 3) broadly triangular; annuli without teeth or hairs; basal 6 annuli curved dorsally; serrulae lobelike, asymmetrical, with fine subbasal teeth.
Male. Not seen. Described as similar to the female and male genitalia illustrated by
Trichiocampus pruni 1 Dorsolateral view; left line points to basal anal cell; right line to position of M joining Sc+R 2 Sheath and ovipositor 3 Lancet.
“THAILAND Mae Hong Son, Namtok Mae Surin NP, Visitor's center, 19°21.593'N, 97°59.254'E , 228 m, Malaise trap, 19–26.viii.2007, Manu Namadkum leg., T5872"; “THAILAND Chiang Mai, Doi Phahompok NP, Doi Phaluang, 20°1.06'N, 99°9.581'E , 1449 m, Malaise trap, 14–21.x.2007, P. Wongchai leg., T6187"; “THAILAND Chiang Mai, Doi Phahompok NP, Doi Phaluang, 20°0.966'N, 99°9.579'E , 1449 m, Malaise trap, 7–14.viii.2007, Komwuan Srisom & Prasit Wongchai leg., T2850" (QSBG, USNM).
This species belongs to the tribe Cladiini, characterized by the forewing venation: vein M meeting Sc+R close to the point where Rs meets Sc+R and vein 2A+3A complete, fused with 1A at its center and forming a basal anal cell. For Cladiini, Benson recorded Priophorus nigricans (Cameron) and Pristiphora brullei Dahlbom from Myanmar, and Cladius pectinicornis (Geoffroy) from the Himalayas. All three have a narrow, well-sclerotized lancet with lateral teeth on the annuli, not the Trichiocampus-like saw as in Fig. 3, which is broadly triangular, lacks annular spines or hairs, and has deep, rounded serrulae.
I refer the Thai specimens to Trichiocampus pruni because of their similarity to the description and illustrations of Trichiocampus pruni provided by
I consider Trichiocampus as a valid genus, following
urn:lsid:zoobank.org:act:F3422910-6C2D-431D-929A-411D54CFEF8
http://species-id.net/wiki/Dineura_sharkeyi
Figs 4–7Female. Length, 4.5 mm. Head black with clypeus brown and mouthparts white. Thorax black with posterior angles or pronotum and tegulae white; small orange-brown spot on central posterior margin of mesepisternum. Legs white with femora light orange. Abdomen brown, lighter than thorax, with narrow posterior margin of segments white; sheath black; 9th tergite, cercus, and apical sternite orange brown. Wings hyaline; veins and stigma brown with extreme bases of veins white.
Head and thorax shiny, with fine white pubescence; abdomen shiny with very fine microsculpture on tergites. Antennal length (apical antennomere missing) about 2.1× head width; 3rd antennomere subequal in length to 4th antennomere. Clypeus circularly emarginated, emargination about half medial length of clypeus. Malar space about 1.5× diameter of front ocellus. Postocellar area 3.6× broader than long. Left mandible evenly tapering from base to apex. Distance between eye and lateral ocellus about 1.2× distance between lateral ocelli. Lower interocular distance 1.6× eye height. In dorsal view, head rounded behind eyes, distance behind eyes about 0.8× eye length. Forewing with 2A+3A straight; 2r present; crossvein 2r-m absent; intercostal crossvein basal to M. Hind basitarsomere subequal to length of following 3 tarsomeres combined. Tarsal claws simple. Tibial spurs short, inner spur about 0.4× length of basitarsomere. Sheath simple (Fig. 6), from above broad at base evenly tapering to acute apex; hairs straight. Cerci shorter than sheath in dorsal view. Lancet long, slender, well sclerotized, serrulae most evident on apical third; annuli with short, stout spines (Fig. 7).
Male. Unknown.
Dineura sharkeyi, holotype 4 Lateral view; line points to vein 2r 5 Dorsal view 6 Apex of abdomen and sheath, dorsal view 7 Lancet.
Female labeled “Thailand, Soi Dao, 500-1850 m., sweep, UTM 1429610, Jan. 16, 2005, Sharkey” (QSBG).
The species is named for the collector, M. J. Sharkey, University of Kentucky.
This species is assigned to Dineura. It shares most characters with species of Dineura except for the simple tarsal claws and absence of vein 2r-m in the forewing: broad malar space, emarginated clypeus, forewing with vein 2r present, intercostal crossvein basal to vein M, and similarities of the sheath and lancet. All other species have the tarsal claw with long inner tooth. The lancet is almost identical to species in Pristiphora subgenus Sharliphora, previously known as the Pristiphora ambigua group. The lancets of the three Pristiphora (Sharliphora) species are illustrated by
Dineura sharkeyi differs from the two species known from China, Dineura blanki Wei and Dineura testaceipes (Klug) by the simple tarsal claws and long lancet with short annular spines. Other species of the genus have a long inner tooth on the tarsal claws and the lancet is shorter and broader and usually with long annular teeth on the central segments (
urn:lsid:zoobank.org:act:7DFF3E30-74B9-400D-A337-1B2EA0FF09C2
http://species-id.net/wiki/Moricella_rueaensis
Figs 8–10Female. Length, 8.0 mm. Antenna black. Head black with clypeus light orange, apex of mandible red brown, labrum and maxilla white to light orange with upper surfaces mostly black. Thorax and abdomen orange; sheath black. Legs orange with apical foretarsomere black, midtarsus brown to black, and apical half of hind femur, tibia, and tarsus black. Wings hyaline; veins and stigma black.
Head smooth, shiny, without punctures. Antennal length 2.3× head width; 3rd antennomere 1.2× length of 4th antennomere. Clypeus short, broad, about 4.0× broader than long, slightly broadly emarginated in front. Malar space short, about 0.25× diameter of front ocellus. Left mandible evenly tapering from base to apex. Distance between eye and hind ocellus subequal to distance between hind ocelli; postocellar area about 2.0× broader than long. Lower interocular distance about 1.1× eye height. Forewing with 3 cubital cells; intercostal crossvein absent; crossvein 2r present in one wing, absent in the other. Pulvilli small, on tarsomeres 1–4. Inner hind tibial spur about ¼ length of hind basitarsomere and equal to width of hind tibia at apex. Hind basitarsomere 1.1× length of remaining tarsomeres combined. Tarsal claws with long inner tooth, equal in length to outer tooth. Sheath from above broad, with short lateral scopae (similar to Benson 1968, fig. 412). Lancet (Fig. 10) with 15 serrulae; annuli strongly curved, V-shaped, with thick, stout spines on dorsal curve and lacking hairs or spines on ventral curve; serrulae with 6 or 7 subbasal teeth on basal serrulae, gradually decreasing to 3 or 4 on apical serrulae; serrulae 2–10 with small spurette dorsal to anterior margin of serrula.
Male. Unknown.
Moricella rueaensis, holotype 8 Lateral view 9 Dorsal view; left line points to position of M joining Sc+R, right line points to vein 2r 10 Lancet.
Female, labeled “THAILAND Loei, Phu Ruea NP, Pah Lo Nay, 17°30.502'N, 101°20.868'E , 1343 m, Malaise trap, 26.ix–2.x.2006, Nu Koonchal Jaaroenchal leg., T833” (QSBG).
The species name is derived from the type locality, Phu Ruea National Park.
Moricella is close to Mesoneura and Dineura and is characterized by the narrower malar space equal to or less than diameter of the front ocellus, clypeus truncate with slight median depression; pentagonal area obsolete; left mandible in lateral view evenly tapering from base to apex, third antennomere slightly longer than the fourth, forewing with intercostal crossvein present and interstitial with vein M and with four cubital cells, the hind basitarsomere equal to the following three tarsomeres combined, and the tarsal claws cleft with the inner tooth slightly long and broader than the outer tooth. The new species shares most characters with Moricella except the forewing lacks the intercostal crossvein and has only three cubital cells. Because of the similarities, including the presence of spurettes near the serrulae and stout, thick spines on the annuli of the lancet, I place this species in Moricella. Moricella includes two other species, Moricella rufonota
The orange thorax and abdomen and mostly orange legs are diagnostic for Moricella rueaensis. Moricella rufonota is black with the pronotum, mesonotum, and upper half or more of the mesepisternum red and the legs have the coxae white apically, femora black, and tibiae white except for the black apical quarter of the hind tibia.
urn:lsid:zoobank.org:act:058DF73A-3702-41E2-AB39-CA729691B715
http://species-id.net/wiki/Nematus_soidaoi
Figs 11–13Female. Length, 7.5 mm. Antenna and head black; spot at center of clypeus, labrum, and mouthparts white. Thorax black with posterior half of pronotum and tegula white. Abdomen and sheath black. Foreleg mostly white, coxa at apex, trochanter, femur except basal and apical parts black; midleg mostly white, coxa at apex and apex of tibia and apical 4 tarsomeres brown to black; hind leg with coxa at apex white, femur black with extreme basal and apical parts white; tibia with two-thirds white, apical third black; basitarsomere with basal half white and apical half black, rest of tarsomeres black. Wings hyaline, veins and stigma black.
Head and body smooth, shiny, without punctures; with fine white pubescence. Antennal length 2.3× head width; 3rd antennomere subequal in length to 4th antennomere. Clypeus roundly emarginated anteriorly. Malar space equal to diameter of front ocellus. Left mandible bulbous at base, apical portion slender. Lower interocular distance 1.3× eye height. Distance between eye and hind ocellus 0.7× distance between hind ocelli; postocellar area about 2.6× broader than long. Forewing with intercostal crossvein present, basal to M; 3 cubital cells. Hind basitarsomere about 0.8× length of remaining tarsomeres combined; pulvilli large, almost equal to breadth of tarsomeres, on tarsomeres 1–4. Hind tibia with longitudinal groove on outer surface. Tarsal claws with long inner tooth, slightly shorter than outer tooth. Inner hind tibial spur about half length of hind basitarsomere and slightly longer than apical width of hind tibia at apex. Sheath in dorsal view broad at base with slight, rounded scopae much shorter than central portion, central portion acuminate at apex, with long setae arising from scopae; in lateral view straight above, rounded below (Fig. 13). In dorsal view, cerci equal to sheath length. Lancet (Fig. 13) with serrula long, lobelike, length more than 2× width.
Male. Unknown.
Nematus soidaoi, holotype 11 Lateral view 12 Head, front view 13 Sheath and lancet.
Female, labeled “Thailand, Soi Dao, 500-1850 m, sweep, UTM 1429610, Jan. 16, 2005, Sharkey” (QSBG).
The species name is derived from the type locality.
This species is assigned to Nematus by similarity of the long inner tooth of the tarsal claws, emarginated clypeus, and similar wing venation. I have not seen any species of Nematus with such long, lobelike serrulae (Fig. 13). The basal rounded portion of the sheath with long setae representing small lateral scopae (as can be seen in the lateral view in Fig. 13) is also unusual for species of Nematus, most of which have an evenly slender sheath in dorsal view. Because the lancet is partly exerted and the long serrulae are diagnostic, I have not dissected the specimen for a close-up of the lancet.
This is a large Holarctic genus with well over 100 species (
(
The pallidiventris complex of
The chlorea complex of
Pristiphora oligalucina Wei, 2002a (China: Henan), is said to have no annular hairs (
Both Pristiphora chalybeata Benson and Pristiphora rufocincta Benson have the abdomen red or yellow; the former has the third abdominal segment red in the female but the male recorded below has identical male genitalia as
urn:lsid:zoobank.org:act:058DF73A-3702-41E2-AB39-CA729691B715
http://species-id.net/wiki/Pristiphora_ettera
Figs 14, 21, 32, 33Female. Length, 7.0 mm. Antenna black; scape and pedicel white. Head black with labrum and palpi white. Thorax black; tegula, pronotum, and postspiracular sclerite (except anterior margin) white; mesoscutellum white at center, black at sides, scutellar appendage and metanotum pale orange. Abdomen with basal plates black; tergites 4–5 with broad anterior halves half or more black; posterior portion black, 6-9 with very narrow posterior portion white; sheath black. Legs white, only apical tarsomeres black. Wings hyaline, veins and stigma black.
Head and body shiny, with fine, minute punctures; covered with fine, white pubescence. Antennal length 2.3× head width; 3rd and 4th antennomeres subequal in length. Malar space linear. Distance between eye and hind ocellus subqual to slightly shorter than distance between hind ocelli. Postocellar area 2.4× broader than long. Lower interocular distance slightly less than eye height. Forewing with 4 cubital cells; intercostal crossvein present and interstitial with M. Pulvilli small; only evident on tarsomeres 3 and 4. Hind basitarsomere subequal to slightly shorter than length of remaining tarsomeres combined; inner hind tibial spur about 0.4× length of basitarsomere and subequal to width of hind tibia at apex. Tarsal claws with long inner tooth, about equal in length and width of outer tooth. Sheath broadened at apex, with distinct rounded scopae (similar to
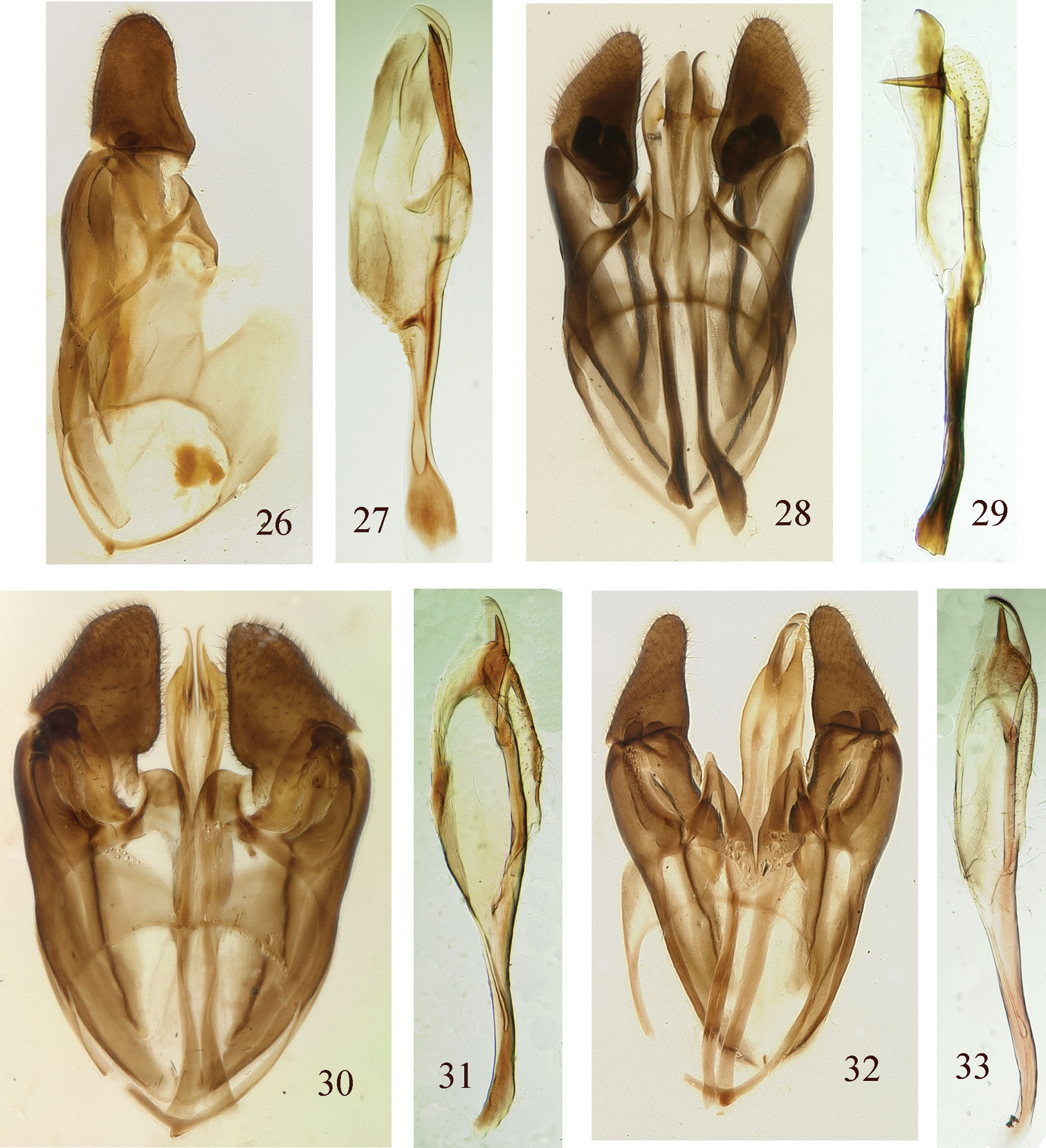
Male. Length, 6.5 mm. Similar to female except head with clypeus and spot on lower inner orbit white; pronotum black with posterior edge narrowly white; and mesoscutellum black. Malar space about half diameter of front ocellus. Lower interocular distance about 1.1× eye height. Forewing with intercostal crossvein present and basal to vein M; with 3 cubital cells. Genitalia in Figs 32, 33; penis valve slender, with a short small valvispina directed vertically near center of valve.
Pristiphora, holotypes 14 Pristiphora ettera, lateral view 15 Pristiphora inthanoni, lateral view 16 Pristiphora inthanoni, dorsal view.
Holotype female, labeled “THAILAND Chiang Mai, Huai Nam Dang NP, Helipad, 19°18.33'N, 98°36.289'E , Malaise trap, 14–21.xi.2007, Anuchart & Thawatchai leg., T5550" (QSBG). Paratypes: “THAILAND, Han Doi Kha NP, Office 12, 19°12.138'N, 101°4.711'E , 1331 m, Malaise trap, 8–15.xi.2007, Chavoen & Nikom leg., T3261" (1 ♀); “THAILAND Chiang Mai, Doi Phahompok NP, Doi Phaluang, 20°1.06'N, 99°9.581'E , 1449 m, Malaise trap, T2928, 20–27.vii.2007, Wongchai P. leg." (1 ♂); “THAILAND, Chiang Mai, Doi Phahompok NP, Kiewlom1/montane forest, 20°3.549'N, 99°8.552'E , 2174 m, Malaise trap, 21–28.v.2008, P. Wongchai leg. T6100" (1 ♂); “THAILAND Chiang Mai, Doi Phahompok NP, Kiewlom1/montane forest, 20°3.549'N, 99°8.552'E , 2174 m, Malaise trap, 21–28.x.2007, P. Wongchai leg. T6181" (2 ♂); “THAILAND Chiang Mai, Doi Phahompok NP, Doi Phaluang, 20°1.06'N, 99°9.581'E , 1449 m, Malaise trap, –14.xi.2007, P. Wongchai leg, T6209" (2 ♂); “THAILAND Chiang Mai, Doi Phahompok NP, Doi Phaluang, 20°1.06'N, 99°9.581'E , 1449 m, Malaise trap, 21–28.xi.2007, P. Wongchai leg., T6211" (2 ♂); “THAILAND Chiang Mai, Doi Phahompok NP, Doi Phaluang, 20°1.06'N, 99°9.581'E , 1449 m, Malaise trap, 21–28.x.2007, P. Wongchai leg., T6188" (1 ♂). (QSBG, USNM).
The species name is an arbitrary combination of letters and is to be treated as a noun.
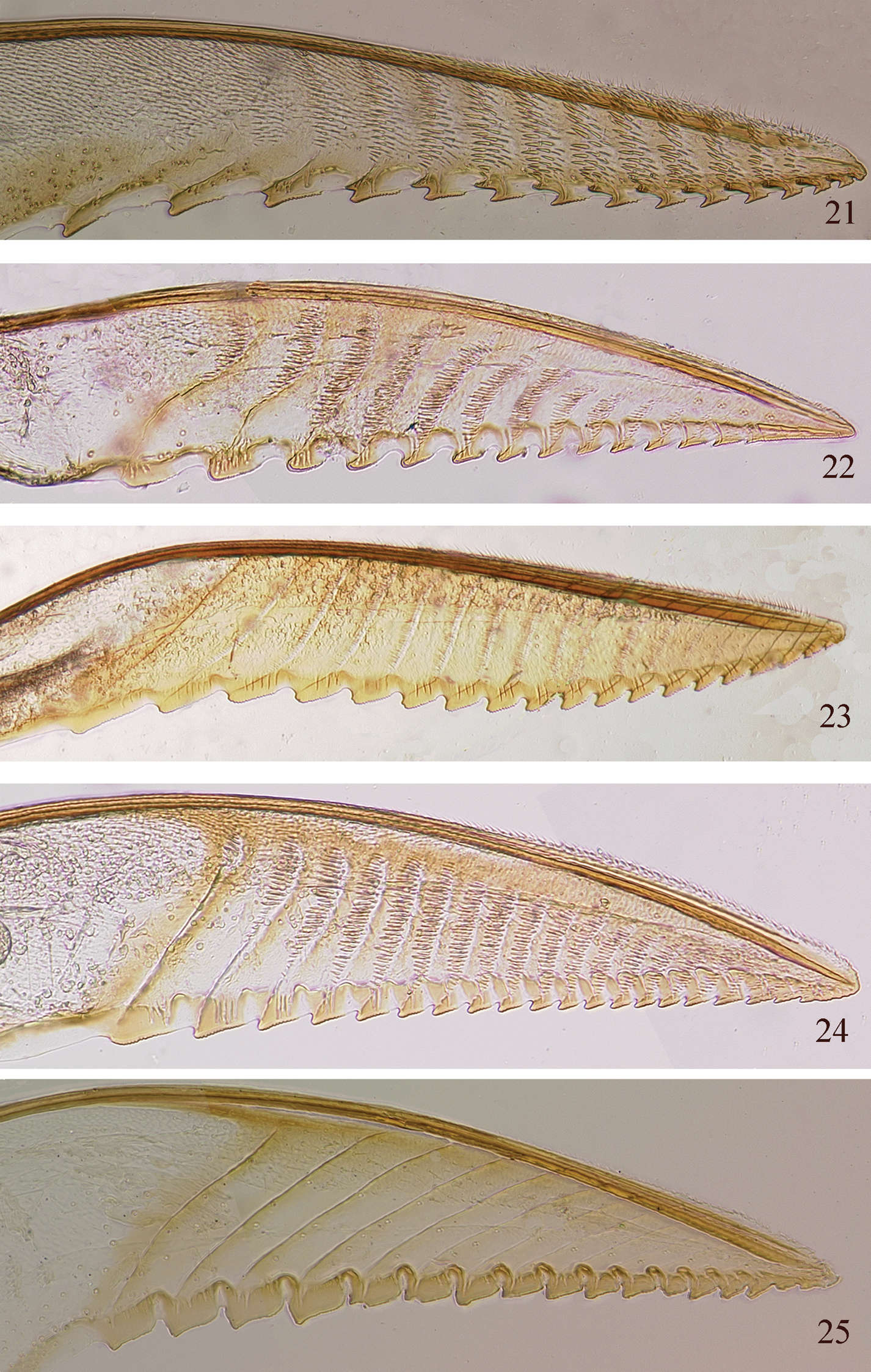
I have not seen other species of Pristiphora with large spurettes above the serrulae plus the strong, stout spines on the annuli (Fig. 21). The only other Pristiphora species illustrated that apparently has such spurettes on the lancet is Pristiphora lineogenata Wei (in
urn:lsid:zoobank.org:act:C1D614DE-036A-4656-9B59-BD9F747D29F8
http://species-id.net/wiki/Pristiphora_inthanoni
Figs 15, 16, 22Female. Length, 5.0 mm. Antenna black, scape and pedicel and undersurface of flagellomeres 1 and 2 brown. Head black with supraclypeal area, clypeus, and labrum brown; mouthparts white. Thorax black with postspiracular sclerite, and tegula white; posterior margin of metapleuron white. Legs white, apical ring on hind tibia black. Abdomen black above; tergites 2 to apex with narrow white posterior band; venter white except apical 3 sternites and sheath black. Wings hyaline, veins and stigma black.
Head and body shiny, finely punctuate, covered with fine white pubescence. Antennal length 2.8× head width; 3rd antennomere equal in length to 4th antennomere. Malar space equal to about half diameter of front ocellus. Distance between eye and hind ocellus about 0.9× distance between hind ocelli. Postocellar area about 3.0× broader than long. Lower interocular distance slightly longer than eye height. Forewing with intercostal crossvein present, interstitial with M; 4 cubital cells (vein separating first two faint). Pulvilli small, on tarsomeres 1–4. Hind basitarsomere subequal to length of remaining tarsomeres combined; inner hind tibial spur about 0.5× length of basitarsomere and slightly longer than width of hind tibia at apex. Tarsal claws with long inner tooth, slightly shorter than outer tooth. Sheath broadened at apex, with rounded distinct lateral scopae (similar to
Male. Unknown.
Female labeled “THAILAND Chiang Mai, Doi Inthanon NP, Checkpoint 2, 18 °31.554'N, 98 °29.940'E, 1700 m, Malaise trap, 24.xi–1.xii.2006, Y. Areeluck leg., T1870" (QSBG).
The species name is derived from the type locality, Doi Inthanon National Park.
The lancet with rounded serrulae at the base becoming flatter toward the apex and absent at the extreme apex, the white legs and venter of the abdomen, and mostly black pronotum are characteristic for this species. It is similar to Pristiphora ettera in color except for the mostly black pronotum and brown clypeus and supraclypeal area. The color is also similar to Pristiphora formosana, but the lancet of Pristiphora formosana lacks annular spines or hairs (
urn:lsid:zoobank.org:act:2C754930-3974-4B0B-AE8E-CBAAECF6C03D
http://species-id.net/wiki/Pristiphora_annetna
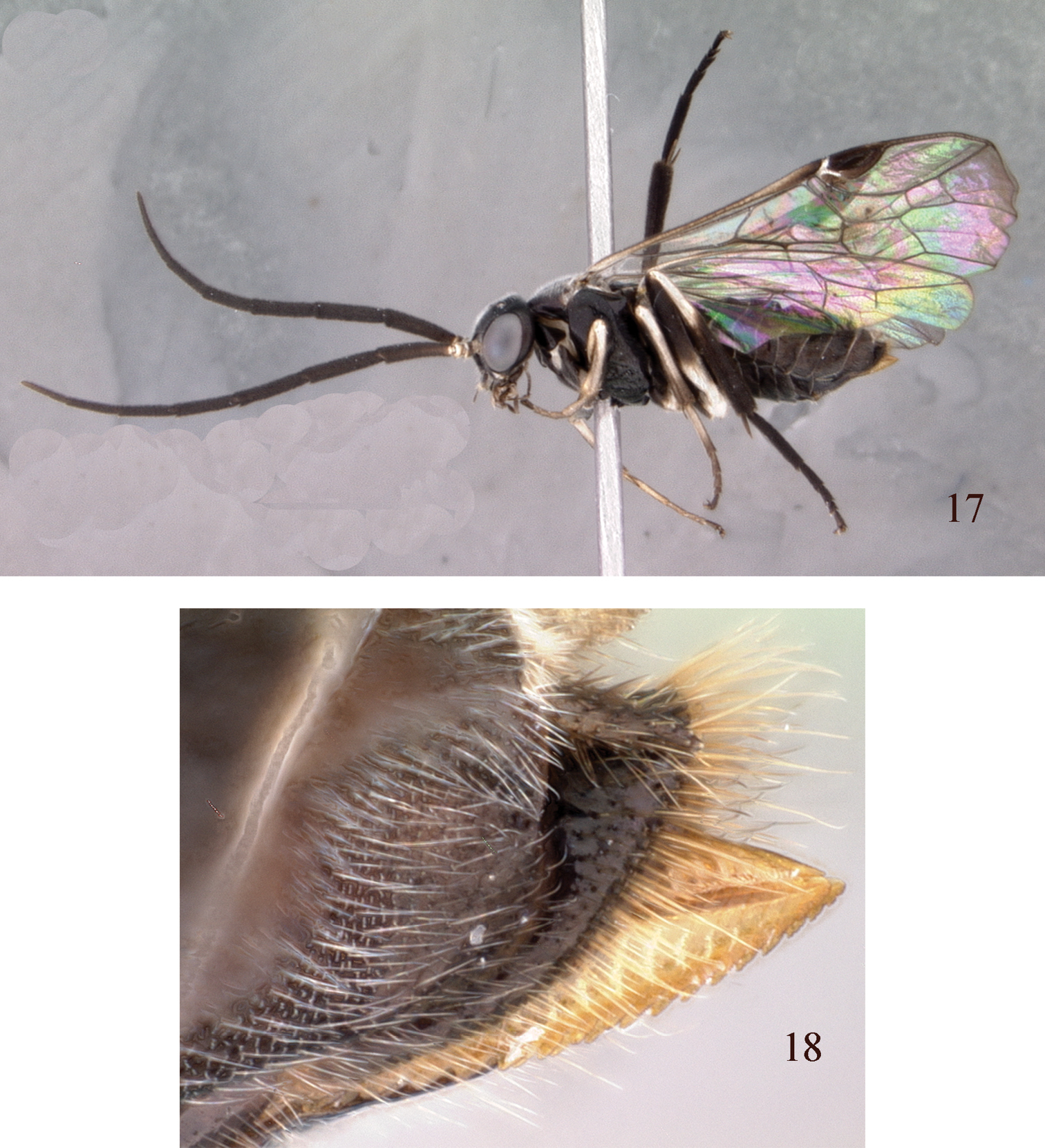
Figs 17, 18, 23, 30, 31Female. Length, 6.0 mm. Black with anterior edge of labrum and mouthparts dull white; scape and pedicel white at apices; tegula and postspiracular sclerite white; apical third of mesoscutellum white. Legs with trochanters white; foreleg white with coxa and inner surface of femur black; midleg with coxa black, trochanter white, femur black, tibia white on basal three-fourths, brown on apical fourth, tarsus with basitarsomeres white to brown with remaining tarsomeres black; hind leg with coxa black, trochanter and base of femur white, basal two-fifths of tibia white, apical three-fifths of tibia and entire tarsus black. Abdomen black. Wings hyaline; veins and stigma black.
Head smooth, shiny, without punctures; head and body densely covered with fine white pubescence. Antennal length 3.7× head width; 3rd antennomere equal to length of 4th antennomere. Malar space about half diameter of front ocellus. Distance between eye and hind ocellus about 0.8× distance between hind ocelli. Postocellar area 3.0× broader than long. Lower interocular distance slightly longer than eye height. Forewing with intercostal crossvein absent; with 3 cubital cells. Pulvilli minute, on tarsomeres 1–4. Tarsal claws with small inner tooth, about half length of outer tooth. Hind basitarsomere subequal to or very slightly shorter than length of remaining tarsomeres combined; hind tibial spur about 0.4× length of hind basitarsomere and equal to width of inner hind tibia at apex. Sheath broadened at apex, with distinct lateral scopae (similar to
Male. Length, 5.0 mm. Similar to female except malar space narrower, almost linear, about ¼ width of front ocellus and postocellar area about 2.2× broader than long. Genitalia in Figs 30, 31; penis valve oval, dorsal and ventral margins rounded, with a short, small valvispina directed vertically near center of valve.
Pristiphora annetna, holotype 17 Lateral view 18 Apex of abdomen, sheath, and lancet.
Holotype female labeled “THAILAND Nan Doi Phu Kha NP, Office 14, 19°12.488'N, 101 °4.907'E, 1375 m, Malaise trap, 22–29.xii.2007, Charoen & Nikim leg., T3280” (QSBG). Paratype: Same data as for holotype (1 ♂, USNM).
The species name is an arbitrary combination of letters and is to be treated as a noun.
This species seems closest to Pristiphora lamdongensis from southern Vietnam, sharing the 3 cu cells and lack of the intercostal crossvein in the forewing and the small inner tooth of the tarsal claws. However, the inner tooth of the tarsal claws is minute in Pristiphora lamdongensis (
urn:lsid:zoobank.org:act:1E7C000E-C40A-4FFF-92DA-12223DCDF1A6
http://species-id.net/wiki/Pristiphora_phahompoki
Figs 19, 20, 24Female. Length 5.5 mm. Black; mouthparts brown. Foreleg white with inner surface of femur black. Mid- and hind legs white; femora and apical third or less of hind tibiae black and apical four tarsomeres of hind leg brown to black. Wings hyaline, veins and stigma black.
Head smooth and shiny, covered with fine, white pubescence, without noticeable punctures. Antennal length 2.3× head width; 3rd antennomere equal in length to 4th antennomere. Malar space about half diameter of front ocellus. Lower interocular distance 1.2× eye height. Distance between eye and hind ocellus 0.8× distance between hind ocelli. Postocellar area about 2.7× broader than long. Forewing with intercostal crossvein present, basal to vein M; with 3 cubital cells. Pulvilli small, on tarsomeres 1–4. Hind basitarsomere slightly shorter than length of remaining tarsomeres combined. Hind tibial spurs about 0.4× length of hind basitarsomere. Tarsal claws with long inner tooth, slightly more than half length of outer tooth. Sheath broadened at apex, with lateral scopae (similar to
Male. Unknown.
Pristiphora phahompoki, holotype 19 Lateral view 20 Dorsal view.
Pristiphora lancets 21 Pristiphora ettera 22 Pristiphora inthanoni 23 Pristiphora annetna 24 Pristiphora phahompoki 25 Pristiphora borneensis.
Female labeled “THAILAND Chiang Mai, Doi Phahompok NP, Kewlom1/montane forest, 20°3.549'N, 99°8.552'E , 2174 m, Malaise trap, 28.ii-7.iii.2008, Seesom. K. leg, T2960" (QSBG).
The species name is derived from the type locality, Doi Phahompok National Park.
This species is mostly black, similar to Pristiphora annetna, but the antennae are short, only about 2.2× the head width, the tegulae are black, the basitarsomeres are white, the forewing has the intercostal crossvein, and the lancet (Fig. 24) has flatter serrulae and longer annular spines. I have not found other species of Pristiphora with the color combination and lancet characters of this species.
http://species-id.net/wiki/Pristiphora_chalybeata
Figs 28, 29 Female. Described by
Male. Length, 5.0 mm. Black with narrow posterior margins of abdominal segments white; legs with coxae black, trochanters white, femora black except extreme apex and base of fore- and midfemora white, tibiae white except apical half of hind tibia black, fore- and midtarsi white with apical 3 tarsomeres black, hind tarsus black with small white spot at base of hind basitarsomere. Wings lightly, uniformly infuscated; veins and stigma black.
Head and body shiny, covered with short white pubescence; head and thorax with widely spaced minute punctures. Antennal length about 2.2× head width, flagellum without thick interspersed spines. Mandible in lateral view swollen at base, with slender bladelike apex. Malar space nearly linear, about one-fourth width of front ocellus. Lower interocular distance about 1.3× eye height. Distance between eye and hind ocellus about 0.9× distance between hind ocelli. Postocellar area about 2.2× broader than long. Tarsal claws with long inner tooth, slightly shorter than outer tooth. Hind basitarsomere 0.8× length of following tarsomeres combined; inner hind tibial spur about 0.2× length of basitarsomere. Genitalia in Figs 28, 29; penis valve slender with strong transverse spine; gonodcardo narrow.
Male genitalia. Genital capsule ventral view; penis valve lateral view 26 Genital capsule, Pristiphora borneensis 27 Penis valve, Pristiphora borneensis 28 Genital capsule, Pristiphora chalybeata 29 Penis valve, Pristiphora chalybeata 30 Genital capsule, Pristiphora annetna 31 Penis valve, Pristiphora annetna 32 Genital capsule, Pristiphora ettera 33 Penis valve, Pristiphora ettera.
THAILAND, Chiang Mai, Doi Inthanon NP, Kew Maepan Trail, 8°33.162'N, 98°28.810'E , 2200 m, Malaise trap, 29.xii.2006–5.i.2007, Y. Areeluck leg., T1893 (1 ♂).
It is possible
http://species-id.net/wiki/Pristiphora_borneensis
Figs 26, 27MALAYSIA: SABAH: British North Borneo, Tenompok, 10–14.II.1959, T. C. Maa, collector (3 ♀, BPBM); British North Borneo, Tenompok, 1460 m, Jesselton, 48 km E., 26–31.I.1959, T. C. Maa, collector (1 ♀, BPBM); North Borneo, Ranau, 22–25.II.1959, T. C. Maa, collector (1 ♂, BPBM).
The male has not been described. It is black with the apex of the fore- and midfemora, fore- and midtibiae and tarsi, and the basal half of the hind tibia white. The lancet was illustrated by
http://species-id.net/wiki/Pristiphora_sinensis
“China, Hupeh, Lichuan Dist., Sui-sa-pa, 27.VII.1948, L. & M. Gressit, collectors” (1 ♀, BPBM).
This species was described from both males and females from Nanking, China. I have seen an additional specimen from China. Wei (in
The National Science Foundation Grant # DEB-0542864, Thailand Biodiversity Inventory (also known as TIGER, Thailand Inventory Group for Entomological Research) to M. J. Sharkey, University of Kentucky, Lexington, is acknowledged. I thank M. J. Sharkey and S. Clutts, University of Kentucky, for sorting and sending specimens. I thank the curator at the B.P. Bishop Museum, Honolulu, HI, for the loan of specimens. Michele Touchet, Systematic Entomology Laboratory, USDA, Washington, DC, assisted with the images. Reviews by the following are appreciated: N. M. Schiff, U. S. Forest Service, Stoneville, MS; D. A. Nickle and T. J. Henry, Systematic Entomology Laboratory, USDA, Beltsville, MD, and Washington, DC, respectively; M. Wei, Central South University of Forestry and Technology, Changsha, China; and an anonymous reviewer. USDA is an equal opportunity provider and employer.