(C) 2012 Erika M. Tucker. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species-group of Camptothlipsis (Hymenoptera: Braconidae: Agathidinae), Lingualongis, endemic to the Western Cape of South Africa is proposed and three new species are described, i.e., Camptothlipsis lingualongis, Camptothlipsis aagota and Camptothlipsis inertusursus. Phylogenetic analyses support this as a distinct monophyletic group within Camptothlipsis. The phenomenon of elongate mouthparts in Hymenoptera and the unusual multiple evolution of such within the agathidines is briefly discussed.
Agathidinae, lingualongis, long mouthparts, systematics, phylogeny, endemism
Agathidinae (Hymenoptera: Braconidae) is an unusual subfamily of hymenopterans. It is found all over the world in most terrestrial habitats, has widely variable life history traits, and has been used several times for biological control (
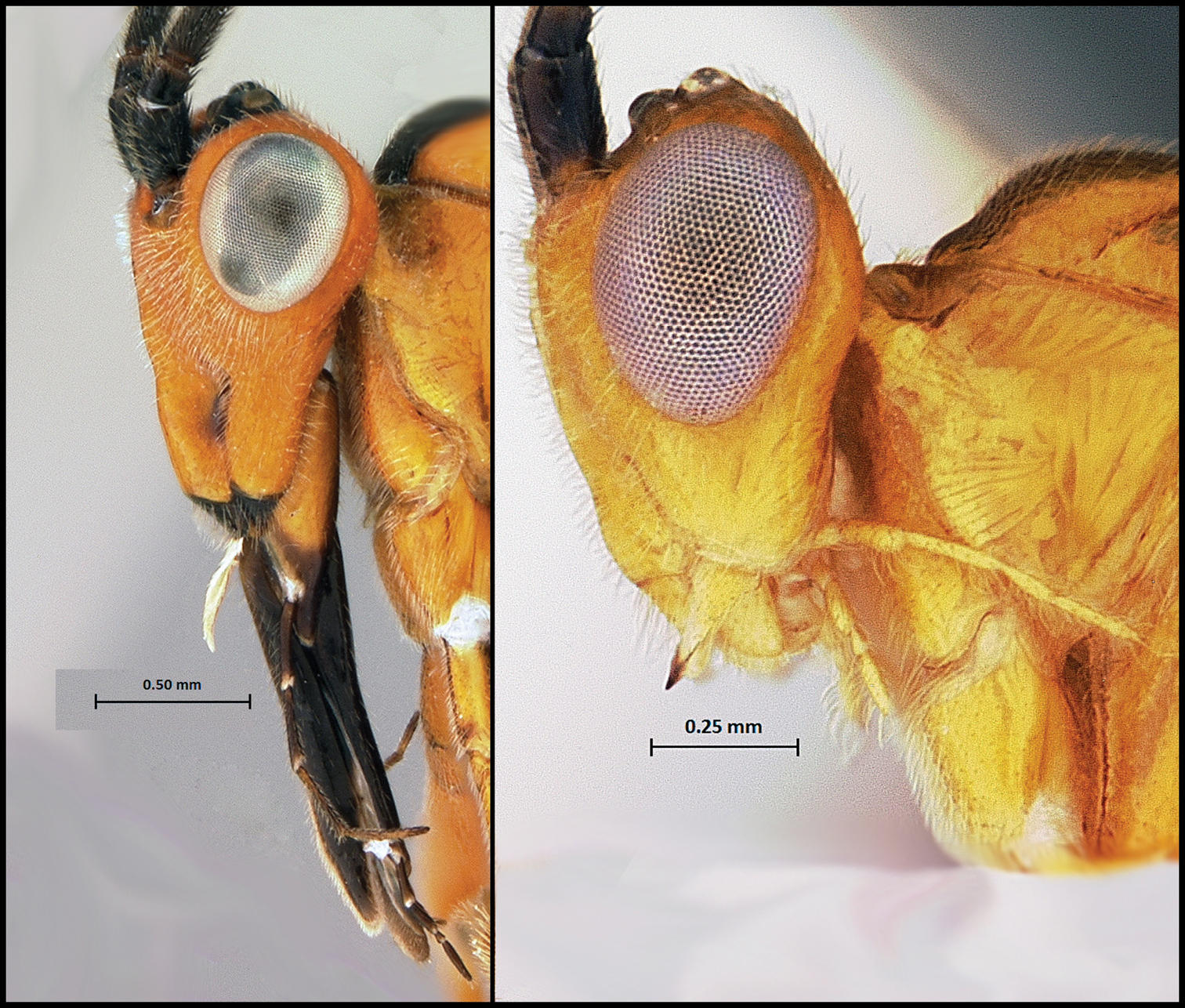
Here we report, a new species-group of Camptothlipsis with elongate mouthparts, the Lingualongis species-group, which is endemic to the Western Cape of South Africa (Fig. 1). Members of the Lingualongis species-group have mouthparts (both maxilla and labium) that are 1 to 1.5 times as long as their heads are high. We suggest two hypotheses in this paper to explain the extreme elongation of mouthpart in this group: 1) they are highly specialized nectar feeders and dependent on the flower of the plant on which their host lepidopterans feed or 2) that the long mouthparts facilitate the extraction of nectar from a wide variety of flowers with both shallow and deep nectaries.
Mouthparts of Camptothlipsis lingualongis, a member of the species-group Lingualongis (left) compared with the mouthparts of a representative of the rest of the genus (right).
The data from the specimens used to produce the cladogram in Fig. 2, with the exception of the new species-group specimen data, are from the data sets of
Most of the material for this study was collected in Malaise traps set up throughout the Western Cape of South Africa, along with a few specimens from sweep samples, with dates ranging from 1997 to 2009. Specimens were sent to the Sharkey Lab in Lexington, Kentucky where they were treated in hexamethyldisilazane (HMDS), mounted, and labeled. Morphological terminology follows
Regions D2–D3 of 28S rDNA were sequenced using the following primers: 28SD2hymF 5’ – AGAGAGAGTTCAAGAGTACGTG – 3’ and 28SD3hymR 5’ – TAGTTCACCATCTTTCGGGTC – 3’. Sequences were edited using Geneious Pro v4.7.5 (
Phylogenetic trees were constructed using maximum parsimony and Bayesian analyses. Maximum parsimony analyses were performed using PAUP (
Each Bayesian analysis consisted of two independent Bayesian runs initiated from different random starting trees. The analysis ran for 2, 000, 000 generations, reaching a topological similarity criterion of 0.005; trees were sampled every 200 generations. 25% of the trees from each run were removed as burn-in upon topological convergence.
See Appendix 1 for the data used to perform this analyses.
Specimen depository codes: HIC: Hymenoptera Institute Collection, University of Kentucky. SAMC: Natural History Department, Iziko South African Museum.
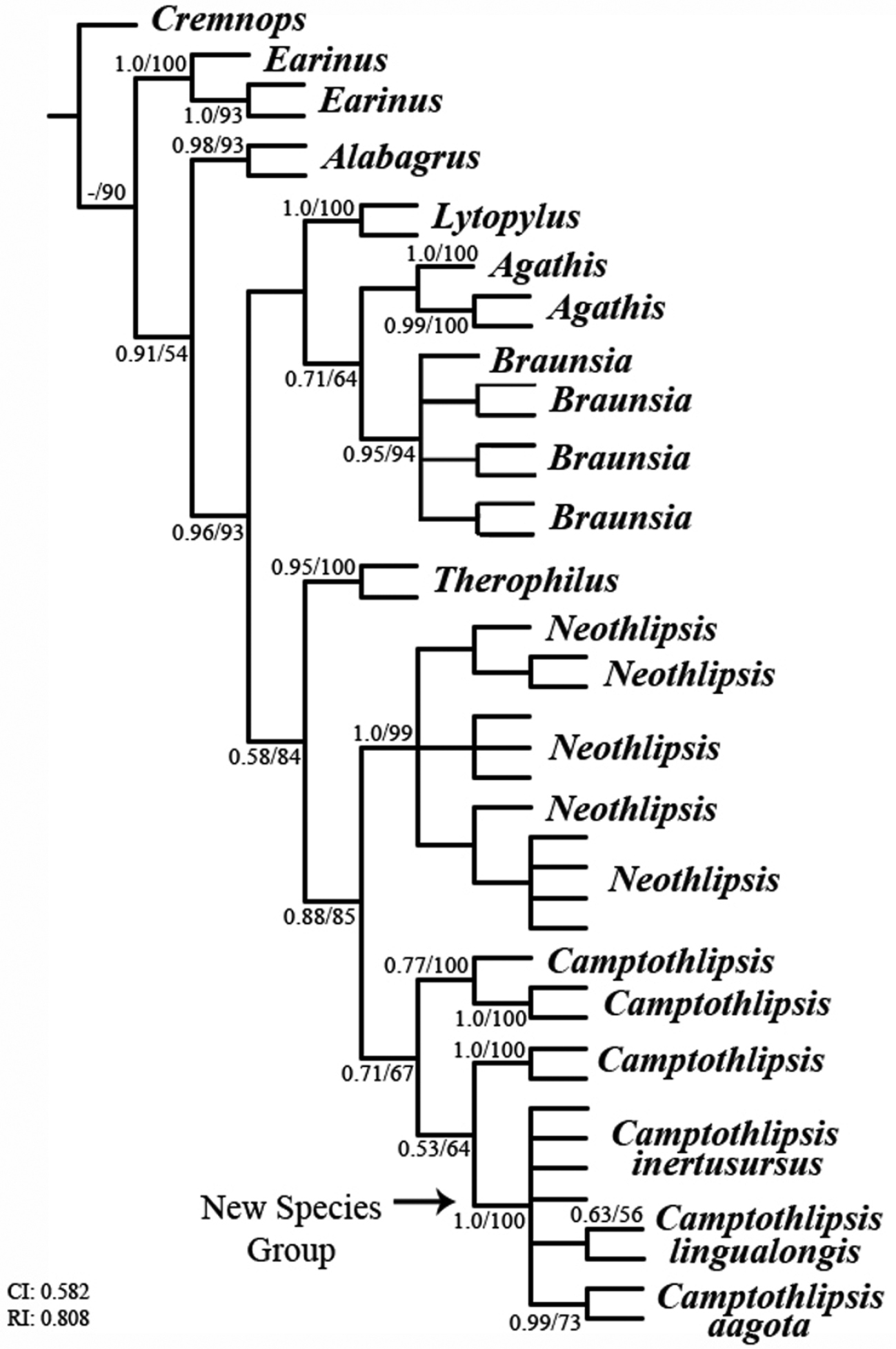
A partial maximum parsimony cladogram of the subfamily Agathidinae. Consistency (CI) and retention (RI) index values are given at bottom left; branch support values are posterior probabilities/bootstrap values.
The maximum parsimony and Bayesian analyses produced almost identical cladograms (the Braunsia clade varying with more or less polytomies). Branch support values (bsv) for the phylogenetic analyses are given as Bayesian posterior probabilities and parsimony bootstrap values, respectively, in Fig. 2, e.g., 63/56. Within the Lingualongis species-group, three different species are indicated in the molecular analysis, i.e. Camptothlipsis inertusursus, Camptothlipsis lingualongis, and Camptothlipsis aagota. Camptothlipsis aagota is strongly supported molecularly (bsv 0.99/73), and morphological characters (deep, foveolate notaulus, long foveolate sternaulus, deeply punctuate propodeum and granulate first tergite) suggest further evidence for the species. Camptothlipsis inertusursus and Camptothlipsis lingualongis are more weakly supported as species molecularly (bsv -/- and 0.63/56 respectively), but distinct morphological characters (very long malar space, foveolate sternaulus, rugose propodeum, and smooth first metasomal tergite, vs. short malar space, smooth notauli, smooth sternaulus, and weak propodeal texturing, respectively) suggest independent species.
The clade containing the Lingualongis species-group is strongly supported molecularly (bsv 1.0/100) and morphological characters (distinctive long mouthparts) suggest further evidence for the species-group. Phylogenetic analyses place the new species-group within the genus Camptothlipsis (Fig. 2). However, since the support values that suggest this placement are rather weak (bsv 0.53/64), more molecular markers are necessary to falsify the hypothesis that the Lingualongis species-group does not deserve generic status as the sister clade to Camptothlipsis.
While almost 60 specimens were tested for viable DNA, only eight 28S sequences were obtained. The degradation of viable DNA could be due to unfavorable conditions, e.g. extreme heat, and/or improper storage conditions before specimens were obtained by the Sharkey Lab. Although not much can be done about the former, if the later is the problem, this can be avoided in the future by proper training and instruction to those tasked with storing specimens before distribution. Due to this inability to obtain viable DNA, and thus 28S sequences, from a larger range of specimens and the high variability of morphological characteristics, only three species are described in this paper. All other potential species (we expect there are approximately 10 of these) are lumped as “undescribed species” in the key.
TaxonomyFigs 3.a–3.e
Head (Figs 3.c – 3.e) - Lateral carina of frons absent; lateral ocellus raised; interantennal space raised, converging into a single distinct anteromedial carina; antennal depression deep; scape simple; 20–35 flagellomeres, apical flagellomere slightly rounded; gena rounded posteroventrally; malar length slightly shorter to 1½ times as long as the height of the compound eye in lateral profile; anterior tentorial pits deep and distinct; mandible with two teeth; galea 1.0–1.5 times as long as height of head; maxillary palpus five segmented, the fourth segment somewhat reduced, length of palp as long or longer than galea; labium slightly longer than galea, and slightly longer than head to 1.5 times longer; labial palpus four segmented, third segment highly reduced.
Mesosoma - Subpronopes separate, and shallow; notauli present; scutellar sulcus present, with numerous longitudinal carinae; metanotum smooth or with carinae; propodeum lightly granulate to deeply pitted to rugose; mesopleuron with mesopleural groove (sternaulus) present; metapleuron slightly rugose ventrally with a rounded point anteroventrally.
Metasoma - Median tergite 1 lacking pair of longitudinal carinae, distal width roughly 1 to 2 times basal width; median syntergum 2 + 3 smooth, with transverse depression separating terga 2 and 3; ovipositor length as long as metasoma + most of mesosoma to as long as entire body.
Wings (Fig. 3.a) - Fore wing: second submarginal cell absent; m-cu broken; 3RSb straight to slightly sinuate. Hind wing: R1 and r-m present; RS nebulous or spectral and sinuate; cu-a not tubular throughout, broken; CUb present as a nebulous or spectral vein.
Legs (Fig. 3.b) - Tarsal claws simple with a pointed basal lobe and 3–5 basal pectines; mid tibia with apical and sometimes subapical spines; hind tibia with apical spines.
Unknown. Presumably they are koinobiont endoparasitoids of lepidopteran larvae. More specifically, caterpillars of the family Gelechiidae, many of which are agricultural pests, are good candidates for this group’s hosts because, to date, the only host records for members of the genus Camptothlipsis are on Gelechiidae larvae (
South Africa, Western Cape.
From the Latin, lingua (tongue), and longis (long). The name refers to the very long mouthparts of this species-group.
Within this species-group the morphology and color are highly variable. The malar length range from slightly shorter to much longer than the height of the compound eye; the anterior face of the head ranges from wider than long to longer than wide; the notauli ranges from V-shaped to Y-shaped, distinctly foveolate to completely smooth; the scutellar sulcus is partitioned into 5 to about 9 sections. These can be almost rectangular to round; the lateral metanotum ranges from smooth to ridged; the sternaulus ranges from ½ to ¾ the length of the mesopleuron, straight to wavy or curved, and can be foveolate to completely smooth; the propodeal sculpture ranges from almost smooth with some slight granulation, to deeply pitted, to rugose; the first metasomal tergite ranges from square-shaped to distinctly elongate, and although the sculpture is usually granulate, it can also be slightly rugose with some specimens being completely smooth. Body length ranges from 2mm to 8mm. Color ranges from completely black to completely orange-yellow with every combination in-between. Three species are described in this paper; however, there are most likely many more species in this species-group that remain to be described.
Lingualongis species-group a wings b hind claw c anterior head d mouthparts e mandible.
| 1 | First metasomal tergite smooth, malar space distinctly longer than eye height; body mostly orange (Fig. 1a and 1aa) | Camptothlipsis lingualongis |
| – | First metasomal tergite granulate, malar space about equal or shorter than eye height; body not mostly orange (Fig. 1b and 1bb) | 2 |
| 2 | Notauli and sternaulus smooth, malar space shorter than eye height, body mostly black (Fig. 2a and 2aa) | Camptothlipsis inertusursus |
| – | Notauli and/or sternaulus foveolate, malar space variable, body color variable (Fig. 2b and 2bb) | 3 |
| 3 | Propodeum deeply punctate, notauli and sternaulus foveolate, and malar space about equal to eye height (Fig. 3a) | Camptothlipsis aagota |
| - | Not fitting the previous couplet descriptions (Fig. 3b and 3bb) | undescribed species |
Descriptions are of the holotype, with species’ variation given within parentheses, e.g., 33 (31–35).
urn:lsid:zoobank.org:act:D9B7511B-DC36-453A-BCE3-CD6AB0BA3BFD
http://species-id.net/wiki/Camptothlipsis_lingualongis
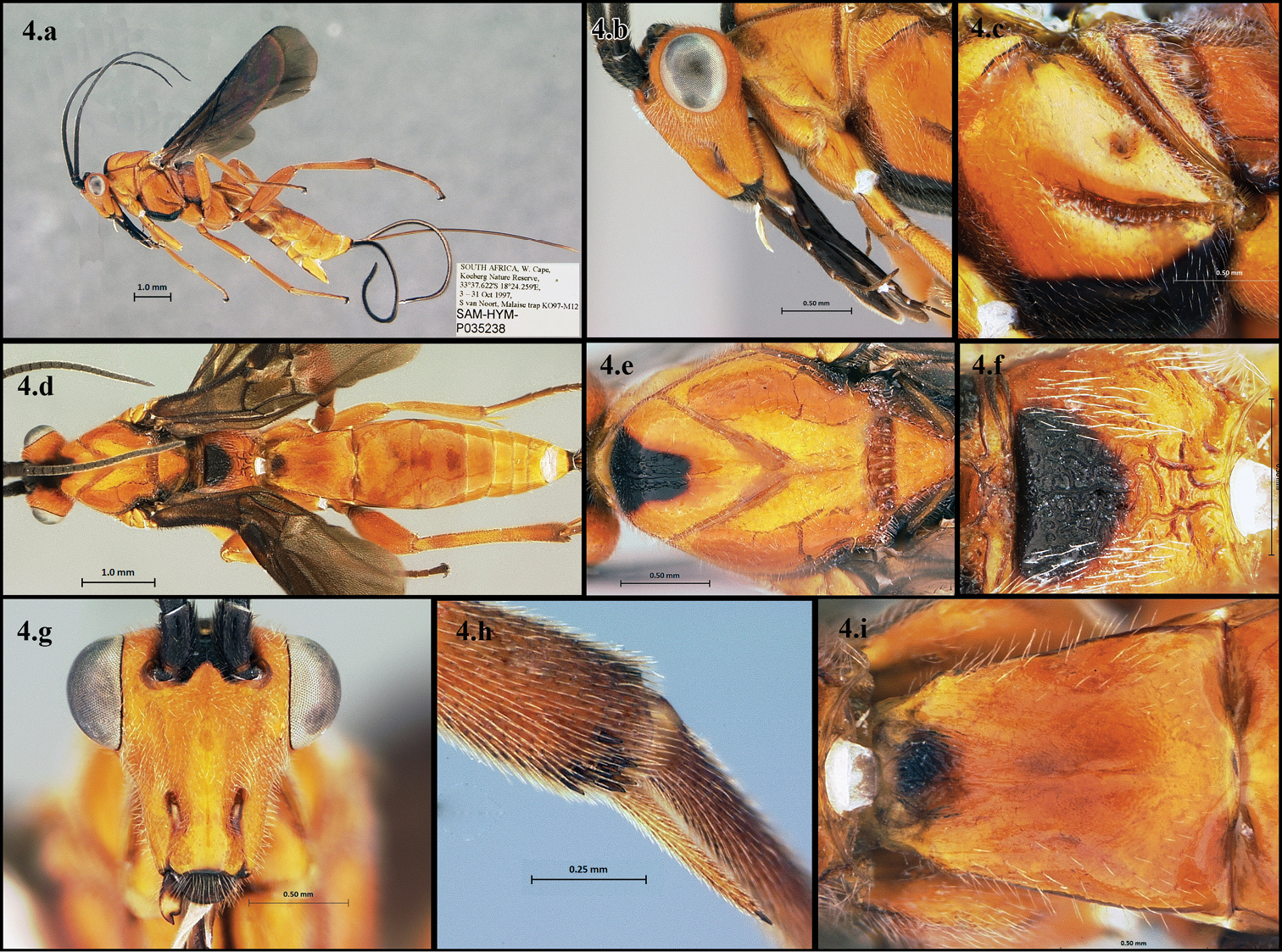
Figs 4.a–4.iThis is the largest and most distinctive species in the Lingualongis species-group. It is easily distinguished by its very long malar space, foveolate sternaulus, rugose propodeum, and its smooth or almost smooth first metasomal tergite (Figs 4.a – 4.i). It is also one of the few members of the group that is all or almost all orange.
Holotype female; length: 7.5mm (4.7–7.5mm).
Head (Figs 4.b, 4.g) – 33 (28–33) flagellomeres; malar length about 1.1× eye height; glossa as long as head height (3.3 vs. 3.3mm); anterior face about as wide as high; ventral-most anterior edge of face shorter than greatest interantennal space; lightly covered with pale setae.
Mesosoma (Figs 4.c, 4.e, 4.f) - Median mesonotal lobe smooth except for faint beginnings of a carina anteromedially; notauli smooth, slightly extended posteriorly after joining medially; scutellar sulcus partitioned into 7 sections by longitudinal carinae; metanotum almost smooth, very faintly rugose; propodeum distinctly rugose, becoming almost areolate rugose posteromedially; sternaulus about ¾ length of mesopleuron, foveolate and slightly sinuate; metapleuron with some carinae, but mostly smooth, and lightly to densely covered with pale setae.
Metasoma (Fig. 4.i) - First median tergite slightly longer than wide (1.2mm vs. 0.96mm), apical width 2× basal width, sculpture smooth with some very faint beginnings of rugosity basally; ovipositor as long as mesosoma + metasoma.
Legs (Fig. 4.h) - Mid leg with 2 apical spines; hind femur length about 3.5× its width at widest point, hind tibia with 7 (7–10) apical spines, entire leg covered with pale setae.
Color - Orange-yellow except black as follows: median vertex of head, median mesonotal lobe apicomedially, posterolateral edges of mesoscutum, most of metanotum, apicomedial part (to all) of propodeum, 1st metasomal tergite basomedially, apical most part of last tergite, labrum and mouthparts, mesosternum and ovipositor sheath; antenna and wings brown, wing veins slightly darker brown.
From the Latin, lingua (tongue), and longis (long). The name refers to the long mouthparts.
JN564494; JN564495.
HOLOTYPE: female, South Africa: Western Cape: Koeberg Nature Reserve, 33°37.62'S, 18°24.26'E, 3–31 Oct. 1997, S. van Noort, Malaise trap KO97-M12 (SAMC). PARATYPES: 1 female, South Africa: Western Cape: Kogelberge Nature Reserve, 34°16.48'S, 19°01.03'E, 16 Nov – 16 Dec 1999, S. van Noort, Malaise trap, KO98-M48 (HIC). 1 female, 2 males, South Africa: Western Cape: Mt. Rochelle, 5km S. of Franschoek, sweep, 10.i.2006, M. Buffington (HIC and SAMC) (accession number JN564495).
Camptothlipsis lingualongis a lateral habitus b lateral head c mesopleuron d dorsal habitus e dorsal thorax f propodeum g anterior head h hind tibial spines i first median tergite of metasoma.
urn:lsid:zoobank.org:act:5515E4C5-1A8E-4F03-9A53-E9E716340FA8
http://species-id.net/wiki/Camptothlipsis_aagota
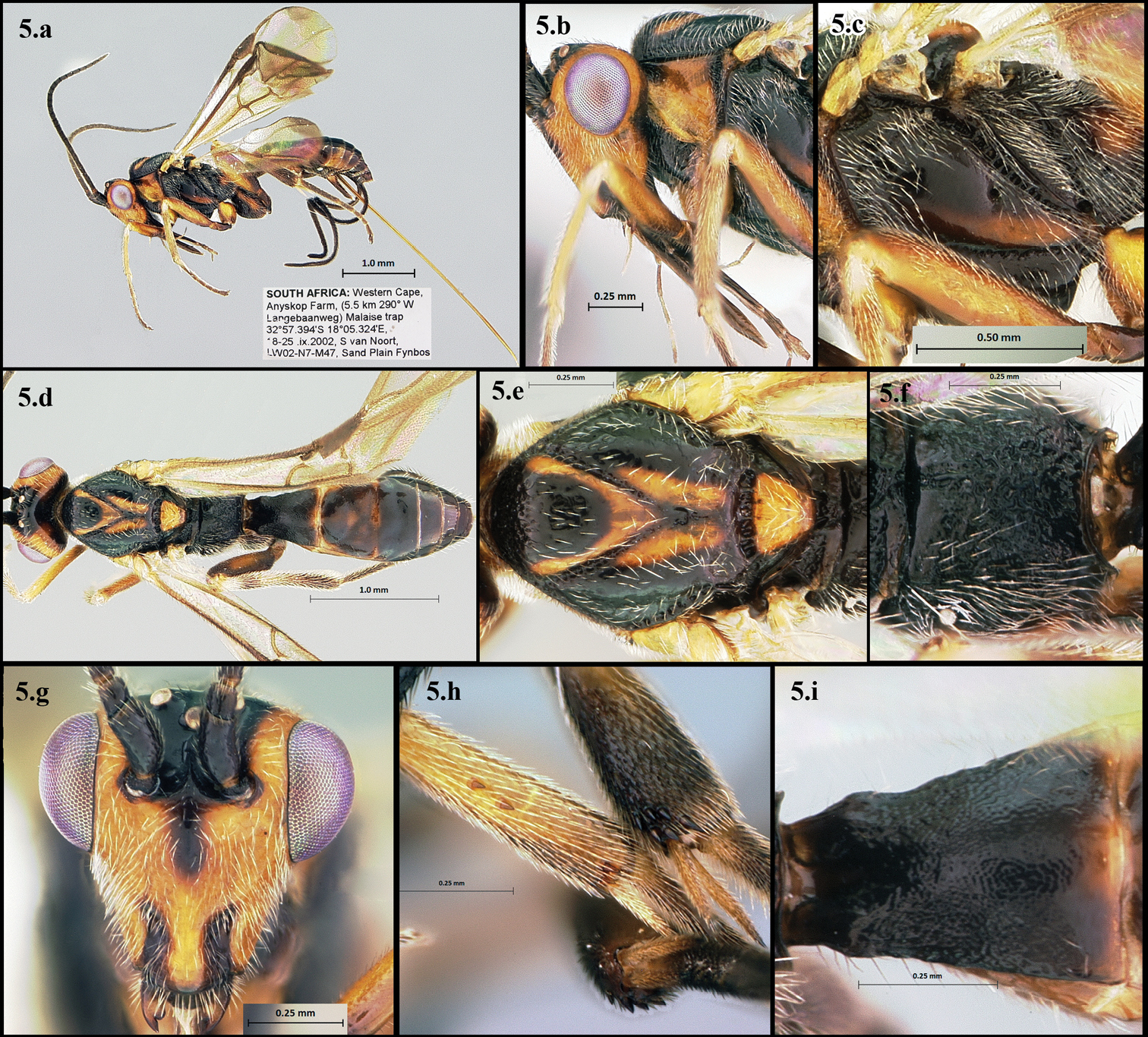
Figs 5.a–5.iThis species is distinguished by its deep, foveolate notaulus, long, foveolate sternaulus, deeply punctate propodeum, and granulate first median tergite (Figs 5.a – 5.i).
Holotype female; length: 3.7mm (3.5–4.5mm)
Head (Figs 5.b, 5.g) – 23 (23–26) flagellomeres; malar length about 1x eye height; glossa slightly longer than head height (0.87 vs. 0.88mm); anterior face about as wide as high or slightly wider (0.83 vs. 0.80mm); ventral-most anterior edge of face shorter than greatest interantennal space; lightly covered with pale setae.
Mesosoma (Figs 5.c, 5.e, 5.f) - Median mesonotal lobe smooth with very faint, shallow pitting; notauli deep and foveolate (sometimes slightly extending posteriorly after joining medially); scutellar sulcus partitioned into 6 (5–9) sections by longitudinal carinae; metanotum very faintly rugose; propodeum moderately to deeply punctate; sternaulus about 4/5 (4/5 -¾) length of mesopleuron, foveolate and slightly sinuate; metapleuron ½ (½-¾) punctate, somewhat densely covered by pale setae.
Metasoma (Fig. 5.i) - First median tergite slightly longer than wide (0.55 vs. 0.46mm), apical width 2x basal width, sculpture granulate; ovipositor as long as entire body.
Legs (Fig. 5.h) - Mid leg with 2 apical and 2 subapical spines; hind femur length about 3× as long as wide, hind tibia with 5 (5–8) apical spines, entire leg covered with pale setae.
Color - Head - Orange-yellow except black as follows: vertex, spot on dorsomedial face, clypeal pit area, labrum, and mouthparts. Antenna and mandible brown. Mesosoma - Black except orange-yellow as follows: pronotum, edges of notauli, two thick medial longitudinal bands on mesoscutum, posteromedial scutellum, longitudinal band above sternaulus, dorsomedial spot on metapleuron. Metasoma - Terga mostly black with some orange-yellow highlights, ovipositor yellow, ovipositor sheath black. Wings - Translucent to pale yellow-brown, veins brown to light brown. Legs - Yellow, except brown as follows: trochanter, basal patch on hind femur, apical tibia and tarsal segments. Hind coxa mostly black.
The species is named after the first author’s mother, Ågot Marga.
JN564488; JN564489.
HOLOTYPE: female, South Africa: Western Cape: Anyskop Farm, (5.5 km 290°W Langebaanweg), Malaise trap 32°57.39'S, 18°05.32'E, 8–25.ix.2002, S. van Noort, LW02-N7-M47, Sand Plain Fynbos (SAMC) (accession number JN564489). PARATYPES: 2 females, same locality data as holotype with dates 9–16.x.2002 and code LW02-N7-M98 (HIC and SAMC).
Camptothlipsis aagota a lateral habitus b lateral head c mesopleuron d dorsal habitus e dorsal thorax f propodeum g anterior head h mid and hind tibial spines i first median tergite of metasoma.
urn:lsid:zoobank.org:act:746C4EC3-62DD-4432-ABCC-FDDF29C77FC5
http://species-id.net/wiki/Camptothlipsis_inertusursus
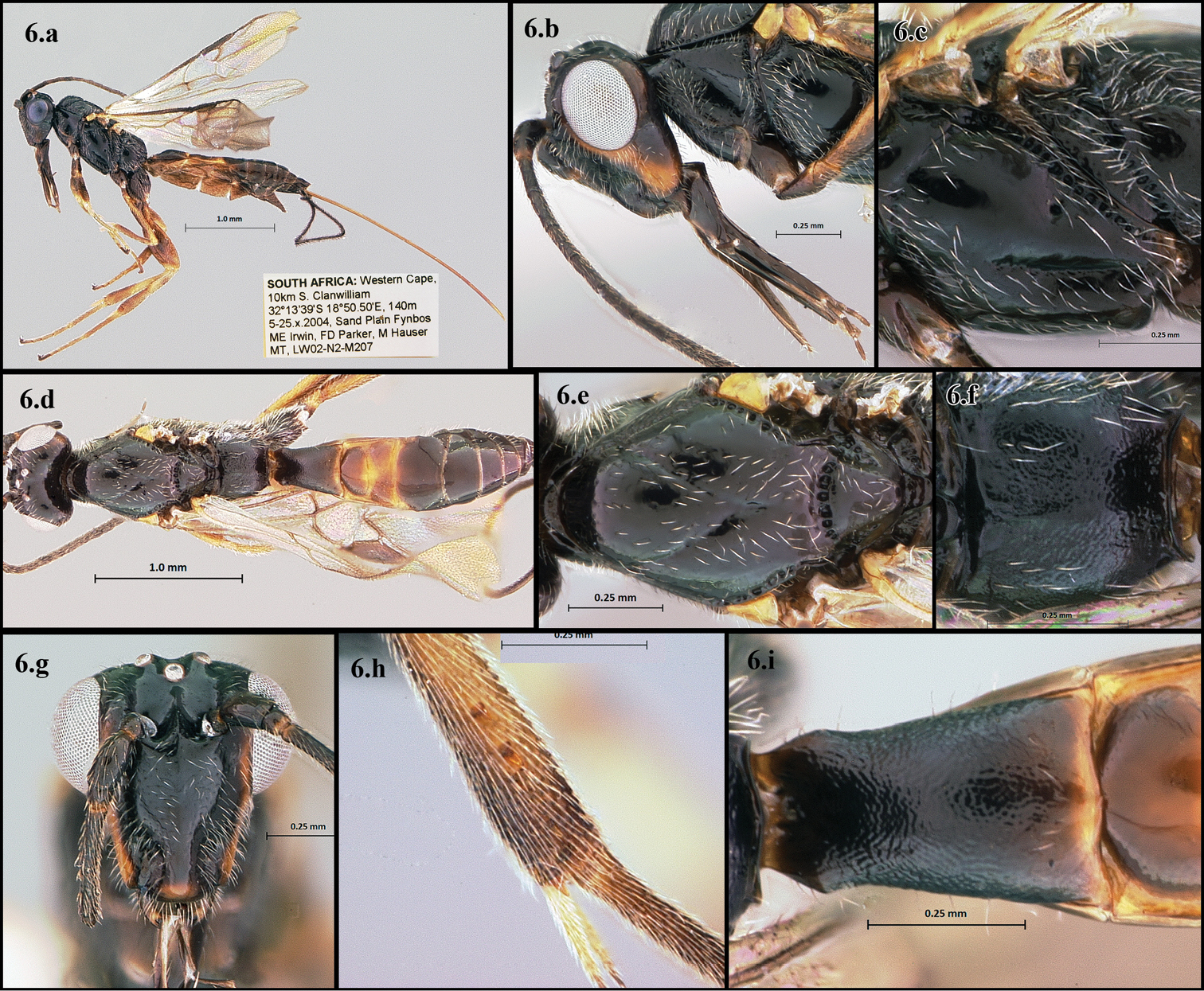
Figs 6.a–6.iThis species is distinguished by its short malar space, smooth notauli, smooth or almost smooth sternaulus, weak propodeal sculpture, and mostly black coloration (Figs 6.a – 6.i).
Holotype female; length: 3.6mm (3.5–4.5mm).
Head (Figs 6.b, 6.g) – 22 (22–27) flagellomeres; malar length shorter than eye height (0.26 vs. 3.6mm); glossa longer than head height (0.80 vs. 0.70mm); anterior face about as wide as high; ventral-most anterior edge of face about same length as greatest interantennal space; lightly covered with pale setae.
Mesosoma (Figs 6.c, 6.e, 6.f) - Median mesonotal lobe smooth; notauli smooth, ending posteriorly where joined medially; scutellar sulcus partitioned into 7 (5–8) sections by longitudinal carinae; metanotum weakly to very weakly pitted; propodeum very weakly rugose, often appearing almost smooth, with a weak anteromedial carina; sternaulus about ¾ length of mesopleuron, smooth to very weakly foveolate; metapleuron mostly smooth with some weak rugosity around the ventral edges, moderately covered with pale setae.
Metasoma (Fig. 6.i) - First median tergite longer than wide (0.45 vs. 0.34mm), apical width slightly less than 2× basal width, sculpture granulate; ovipositor slightly longer than mesosoma + metasoma.
Legs (Fig. 6.h) - Mid leg with 3 (3–5) apical and 2 (2–3) subapical spines; hind femur length about 3x as long as wide, hind tibia with 10 (6–12) apical spines (occasionally 1 subapical spine), entire leg covered with pale setae.
Color - Black except yellow to orange as follows: longitudinal band on malar space, apex of antennal pedicel, base of first flagellomere, labrum, mandible, tegula, legs, 2nd and 3rd metasomal terga and ovipositor, coxa brown-black; wings translucent with brown to light brown veins.
From the Latin inertus (lazy), and ursus (bear). The species is named for the first author’s father, whom she thought would be amused by the name.
JN564490; JN564491; JN564492; JN565593.
HOLOTYPE: female, South Africa: Western Cape, 10km S. Clanwilliam, 32°13.39'S, 18°50.50'E, 140m, 5–25.X.2004, sand plain fynbos, ME Irwin, FD Parker, M Hauser, MT, LW02-N2-M207 (SAMC). PARATYPES: 2 females, same locality and date data as holotype (HIC and SAMC) (accession number JN565593).
Camptothlipsis inertusursus a lateral habitus b lateral head c mesopleuron d dorsal habitus e dorsal thorax f propodeum g anterior head h mid tibial spines i first median tergite of metasoma.
This species-group has type 1 mouthparts, i.e., the glossa extracts nectar and then in retracted within the galeae, which close around it and allow the liquid to be sucked into the alimentary canal (
Our preferred hypothesis is the first and we suggest that, like Agathis malvacearum, members of the Lingualongis species-group may have an obligate and symbiotic relationship with their host flowers, in that the host plant provides nectar and host lepidopterans, and the wasp pollinates and protects the plant by keeping host lepidopteran populations in check.
The Western Cape of South Africa is a biodiversity hotspot and its long term environmental stability allows for the survival of large concentrations of relict species, such as the stag beetle Colophon montisatris Endrödy-Younga, and encourages organisms to radiate and differentiate morphologically (
We would like to thank Simon van Noort and his technicians for sorting and sending us most of the specimens that made this paper possible. This project was funded by the National Science Foundation HymATol project, grant number: EF-0337220.
Data used to perform the analyses. (doi: 10.3897/JHR.24.1909.app1). File format: NEXUS file (NEX).
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.