(C) 2012 Christophe Barthélémy. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of the cryptine genus Hadrocryptus is described based on specimens reared from bamboo trap nests in Hong Kong. Hadrocryptus perforator sp. n. is an ectoparasitoid of aculeate Hymenoptera larvae and prepupae of the families Vespidae (Eumeninae) and Sphecidae. These are the first host records for this genus.
Xenorhynchium, Zethus, Isodontia diodon, taxonomy, host, Sphecinae
The subfamily Cryptinae is the largest subfamily of Ichneumonidae, with about 400 genera described to date (
Hadrocryptus Cameron, 1903 belongs to a group of genera in the tribe Cryptini that are often referred to as the sub-tribe Gabuniina. Where known, gabuniines parasitise wood-boring beetles (
There are six described species of Hadrocryptus, found in India, Bhutan, the Philippines, Indonesia and Malaysia (
Morphological terminology follows
Apical flagellomere with truncate hammer. Face with horn just below and between antennal sockets. Clypeus with median tooth. Mandible with lower tooth longer than upper. Epomia short and weak. Mesoscutum short, slightly wider than long and no longer than area of propodeum behind anterior transverse carina. Groove between metapleurum and propodeum wide, crossed by carinae. Propodeum lacking pleural carina between anterior transverse carina and hind coxa. Propodeal spiracle elongate. Fore tibia swollen. First tergite with pronounced basal, lateral teeth; with elongate, dorsal depression at level of spiracles. Second tergite strongly punctate. Ovipositor lower valve with vertical teeth, dorsal lobe enclosing upper valve. Fore wing vein cu-a far basal to Rs+M. Hind wing with abscissa of Cu between M and cu-a longer than cu-a.
urn:lsid:zoobank.org:act:42AA95D5-15E8-40F5-913C-8A813439968E
Female, exact label data: ‘Pak Sha O, HK 50Q KK 242 849. 70m C. Barthélémy, 22.iii.10 Ref: 0386.A.Hy.1’, ‘Hong Kong Ex Xenorhynchium sp. in bamboo trap nest, coll. 7.2009, em. 3.2010 BMNH(E) 2010-74 AQ’, BMNH. Paratypes: 1 female, same data as holotype but ‘20.iii.10 Ref: 0384.A.Hy.1’, 1 male, same data as holotype but ‘13.vi.10 Ref: 0414.A.Hy.1’ and ‘Ex Isodontia diodon in bamboo nest em. 6.2010’, both BMNH; 1 female same data as holotype, 1 female same data as holotype but ‘20.iii.10, ref.: 0383.A.Hy.1’, 2 males same data as holotype but ‘ 03.iv.11 ref.: 0432.A.Hy.1’ and ‘ Ex. Zethus sp.’, all BPC.
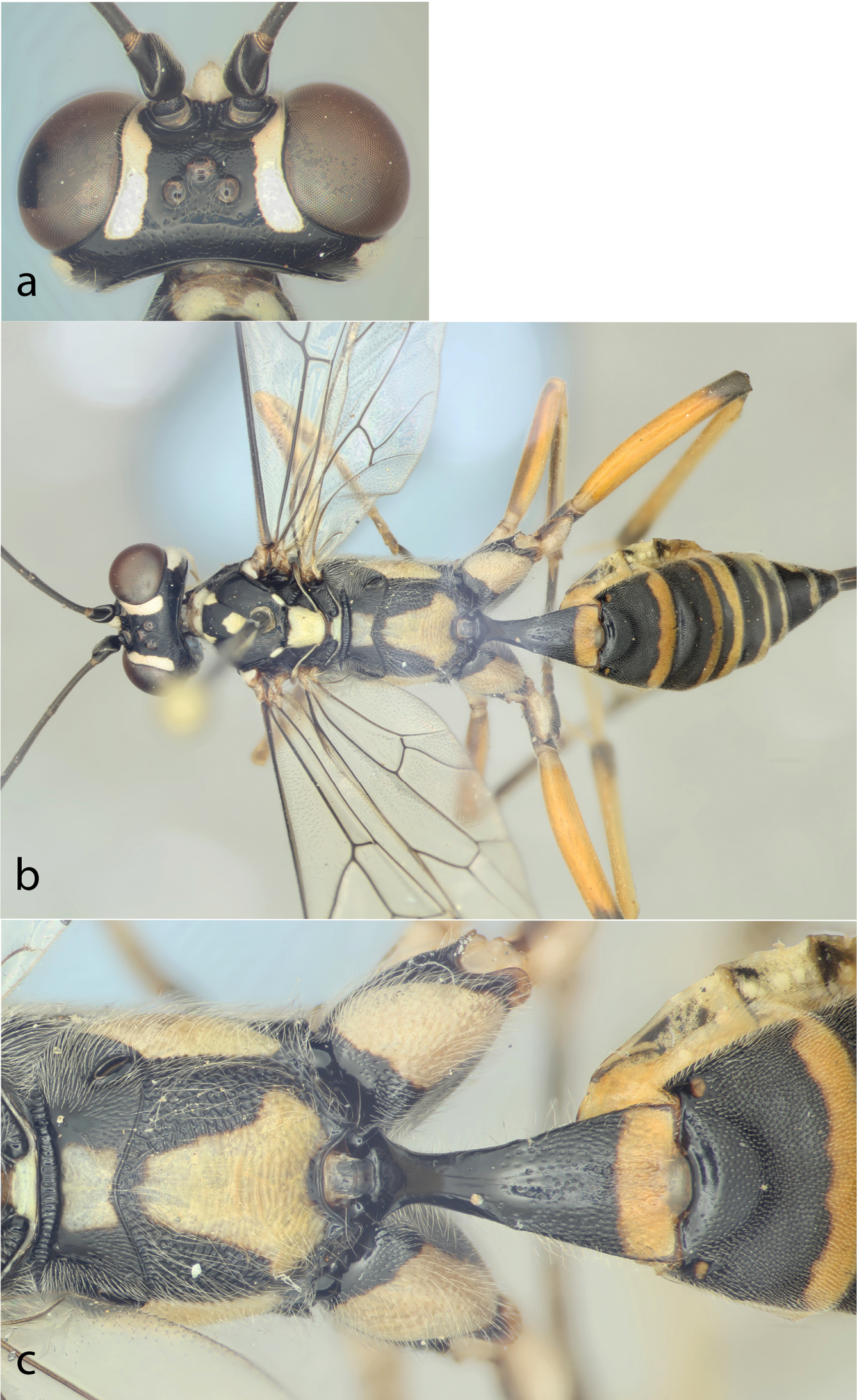
Female. First flagellomere 7.0× as long as apically wide (basal ‘annellus’ section excluded). Frons largely polished, with weak punctures ventral to ocelli and some oblique wrinkling in a V-shape ventral to anterior ocellus. Vertex with conspicuous punctures, separated by more than twice their diameter. Facial horn, just below and between antennal sockets, large, pointed oval in dorsal view, protruding by distance slightly greater than inter-antennal distance (Fig. 1a). Face and clypeus closely punctate, punctures becoming confluent medially on face. Clypeus apically concave with obtuse, low median tooth.
Pronotum mostly smooth, transversely striate lateromedially with short transverse striae along posterior edge. Mesoscutum polished, weakly granulate anteriorly, with isolated punctures anteriorly, medially. Notauli strong to level of anterior edge of notauli, fading then. Scutellum with punctures on smooth background, separated by about their own diameter. Mesopleuron densely punctate, punctures small and separated by about their diameter, except transversely striate anterodorsally and medially, speculum and subalar prominence weakly punctate on smooth background. Metapleuron strongly punctate posterodorsally, sparsely punctate medially. Pleural carina absent except basally. Juxtacoxal carina strong but with narrow interruptions anteriorly and posteriorly. Submetapleural carina complete. Propodeum with area anterior of anterior transverse carina polished, punctate in posterior half; anterior transverse carina strong, complete; remainder of dorsal surface reticulate, transversely striate medially (Fig. 1c). First tergite polished, punctate on apical half, punctures coalescing medially, apically (Fig. 1c). Lower edge of first sternite with weak teeth, separated by more than their width. Second tergite with semicircular basal, median area defined by depression behind this; third tergite with basal area defined by shallower curve; second and third tergites densely punctate, punctures faint on successive tergites. Ovipositor (measured from tip of hypopygium) 2.1× length of hind tibia.
Mainly black with copious ivory/white markings (Fig. 1b). The following ivory: several median flagellomeres, face, clypeus (except for small basal, median black patch), horn on upper face, inner orbits to vertex, outer orbits to dorsal 0.7 of eye, central, median part of pronotum, lateral corners of median lobe of mesoscutum, central area on mesoscutum, tegula, short section of lateral carina of mesoscutum immediately anterior to scuto-scutellar groove, subalar prominence, posteroventral patch on mesopleurum, scutellum, metascutellum, median longitudinal stripe on propodeum, interrupted posterior to transverse carina, widened posteriorly, metapleurum dorsally, posteriorly, fore and mid coxae (except tiny lateral black spot), extensive dorsal, posterior area on hind coxa, all trochanters (with brown dorsal stripe on mid and hind trochanters), fore and mid trochantelli, ventral patch on hind trochantellus, outer surface of fore and mid tarsomeres 1 to 3, all of hind tarsus except for apical patch on 5th, posterior bands on 1st to 7th tergites dull yellow or cream. Fore and mid femora and tibiae orange with dark brown dorsal stripes, fading to cream ventrally, hind tibia orange, apical 0.18 black, hind femur orange, apically and basally black-ringed.
Male. Similar to female, differs (in addition to the obvious sexual differences) only in the more extensive ivory area on the mesopleuron, extending anteriorly as lateral and median stripes on the mesosternum, and in the less modified antenna tip (simply pointed).
Hadrocryptus is an easily recognised genus of the Gabunia genus-group (Gabuniina of
Holotype female of Hadrocryptus perforator sp. n.; a head, dorsal view, showing horn; b whole body, dorsal view; c propodeum and base of metasoma, dorsal view.
Hadrocryptus perforator was reared from six trap nests placed in and collected from three locations in Hong Kong: (1) Ng Tung Chai, UTM 50Q KK 042 840, 140m asl, trap referenced as NTC-028.A4, set adjacent to a healthy 60+ year old forest on the slopes of Hong Kong’s highest peak; (2) Sha Lo Tong, UTM 50Q KK 100 886, 180m asl, trap referenced as SLT-025.A1 and SLT-025.A4 of the same bundle, set adjacent to a Fung Shui wood behind an abandoned village; and (3) Pak Sha O, UTM 50Q KK 242 849, 70m asl, trap referenced as PSO-093.A3, PSO-117.A2 and PSO-117.A6 set in the first author’s garden adjacent to a healthy secondary growth forest. All locations were in shaded environments.
The traps consisted of hollow bamboo canes of varied length and diameter that were cut so that one end was closed by a nodal septum. Clusters of four to seven cut canes were bundled together and hung from low branches on various bushes and trees. Dimensions of traps are given in Table 1
Upon collection, segments were placed in Ziploc® bags and opened in the laboratory for content examination, the data are summarised in Table 2.
| Trap reference | Date set | Date collected | Trap diameter (mm) | Trap length (mm) |
|---|---|---|---|---|
| SLT.025.A1 | 17-Apr-09 | 22-Feb-10 | 10 | 250 |
| SLT.025.A4 | 17-Apr-09 | 22-Feb-10 | 10.5 | 253 |
| NTC.028.A4 | 17-Apr-09 | 10-Jul-09 | 11 | 250 |
| PSO-093.A3 | 12-Apr-10 | 03-Jun-10 | 7.5 | 165 |
| PSO-117.A2 | 23-Jun-10 | 26-Sep-10 | 9 | 225 |
| PSO-117.A6 | 23-Jun-10 | 31-Oct-10 | 8 | 225 |
| Trap reference | Host sub-family | Host species | No. of host cells | No. of cells parasitized | Parasitoid emergence date | Males | Females |
|---|---|---|---|---|---|---|---|
| SLT.025.A1 | Eumeninae | Xenorhynchium sp. | 7 | 3 | 20-Mar-2010 & 22- Mar-2010 | 3 | |
| SLT.025.A4 | Eumeninae | Xenorhynchium sp. | 6 | 1 | 20-Mar-10 | 1 | |
| NTC.028.A4 | Eumeninae | Xenorhynchium sp. | 1 | 1 | 01-Aug-09 | 1 | |
| PSO-093.A3 | Sphecinae | Isodontia diodon | 3 | 1 | 13-Jun-10 | 1 | |
| PSO-117.A2 | Eumeninae | Xenorhynchium sp. | 2 | 1 | ? | ? | ? |
| PSO-117.A6 | Eumeninae | Zethus sp. | 5 | 2 | 3-Apr-2011 & 04- Apr-2011 | 2 | |
| Totals | 24 | 9 | 4 | 4 |
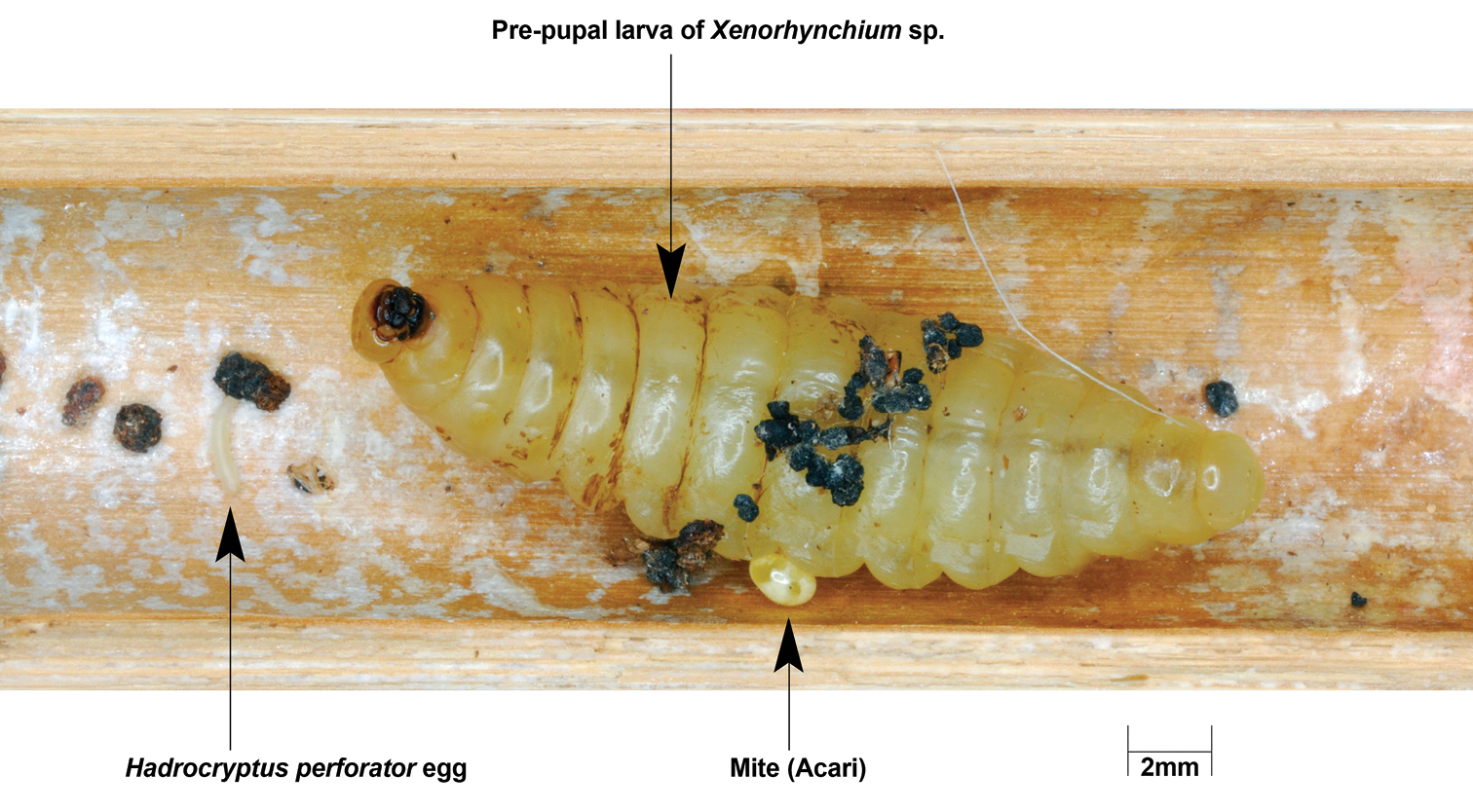
At opening on 10 July 2009, trap NTC-028.A4 contained a single cell with a prepupal larva of Xenorhynchium sp., a gravid female mite (Acari) and a single egg of Hadrocryptus (Fig. 2), laid 4-5 mm from the host prepupal larva. The egg was slightly arched, 2.4 mm long with a more or less constant diameter of 0.46 mm. Upon hatching, the cryptine larva had to reach its host and probably fed externally on it. It took 21 days for the parasitoid to complete development, emerging as a male.
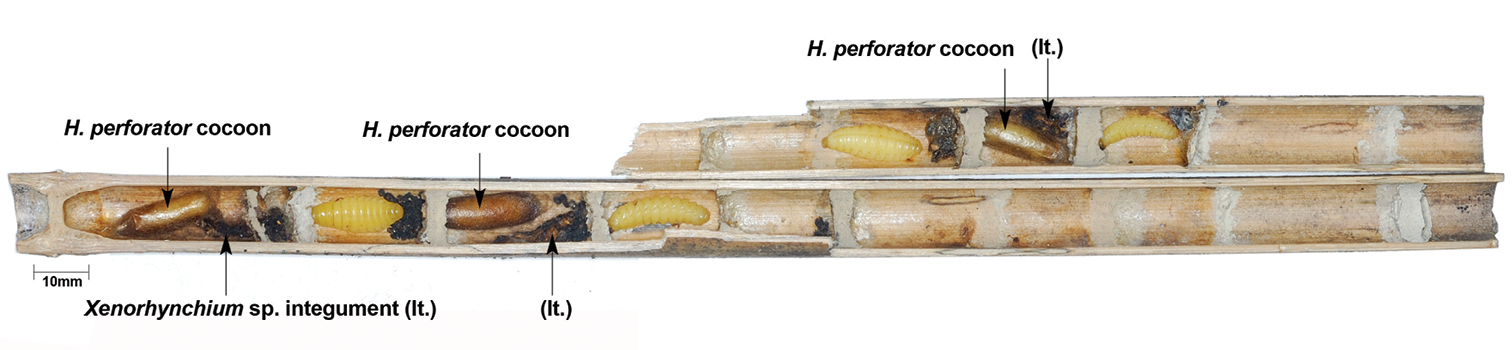
At opening on 22 February 2010, trap SLT-025.A1 contained seven cells with four prepupal larvae of Xenorhynchium sp. (Vespidae: Eumeninae) in cells 2, 4, 5 and 7 and three cocoons of Hadrocryptus in cells 1, 3 and 6 (Fig. 3). The desiccated integuments of the host larvae were also found in the parasitised cells. All three parasitoids were females. The first author witnessed the emergence of Hadrocryptus perforator from cell 1 and it took approximately three minutes for the cryptine to breach its cocoon (Fig. 5); the individual in cell 3 followed minutes afterwards.
Trap SLT-025.A4 contained six cells with five pre-pupal larvae of Xenorhynchium sp. and one cocoon of Hadrocryptus (Fig. 4) in cell 4 which also contained the desiccated integument of the host larva. The single individual emerged as a female on 20 March 2010 when the first author inspected the trap content. The cryptine took approximately five minutes to breach the cocoon.
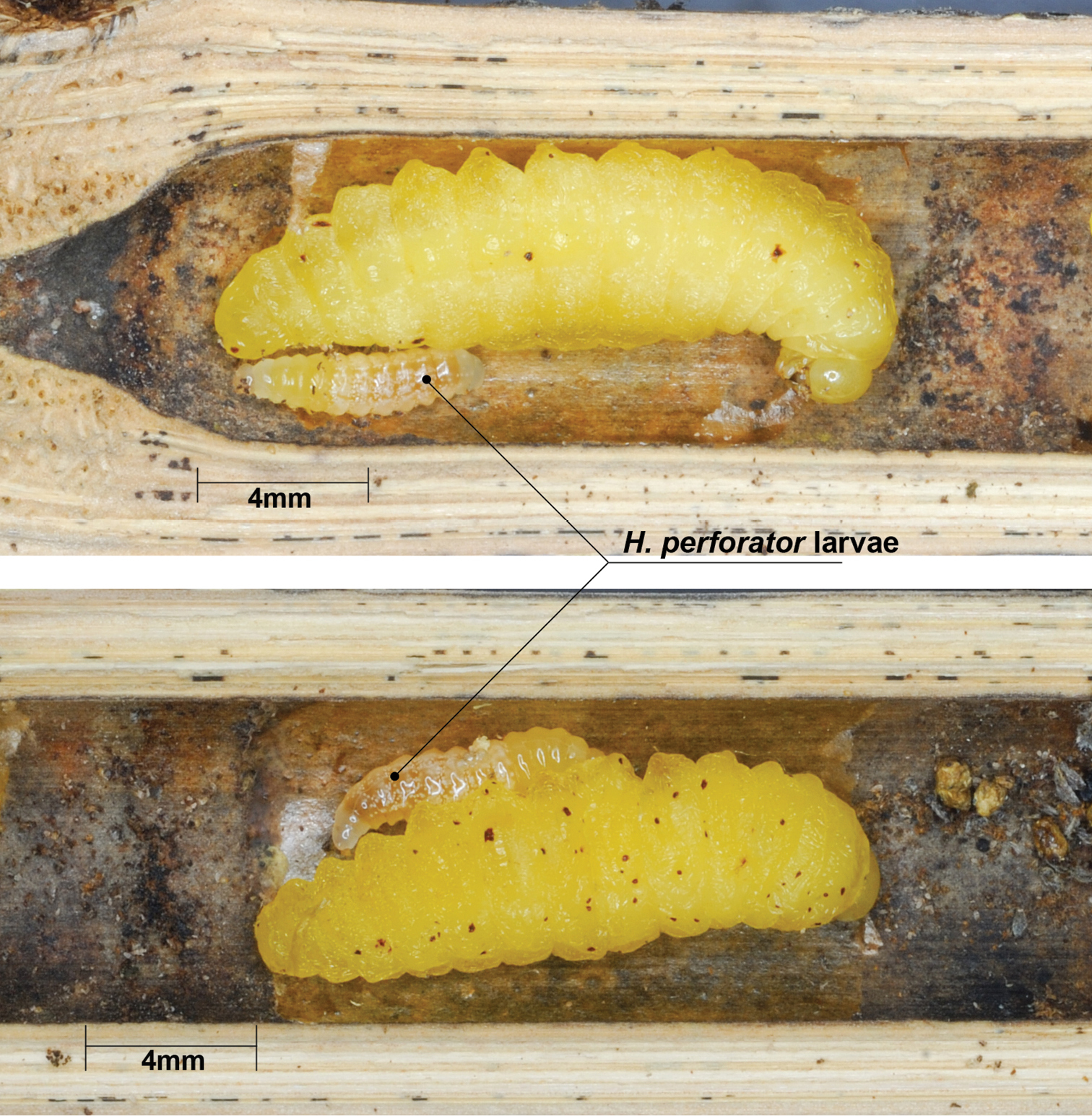
Trap PSO-093.A3 contained three cells fabricated by Isodontia diodon (Sphecidae: Sphecinae), a single male parasitoid emerged. Trap PSO-117.A2 contained two cells of Xenorhynchium sp., of which one was parasitised by Hadrocryptus perforator. Emergence of the parasitoid has not been witnessed at drafting of this paper. Trap PSO-117.A6 contained five cells of Zethus sp. (Vespidae: Eumeninae), of which two were parasitised. At opening there were five prepupal larvae of the host and two small larvae of the parasitoid in cell 1 and cell 3 (Fig. 7). Both emerged as males.
In all cases the parasitoid pupae were positioned head towards the trap entrance. By 14 March 2010, the Hadrocryptus pupae showed clear colour markings through the cocoon in both SLT-025.A1 and A4 traps (Fig. 6).
Four out of six parasitised nests were of Xenorhynchium sp., although a different eumenine was also host to the parasitoid and more surprisingly a sphecid. Data are too limited to say whether there is any taxonomic host preference. With development as an idiobiont ectoparasitoid, it is probable that a variety of aculeate Hymenoptera in stem nests will be suitable as hosts.
The parasitoid’s cocoons were 15.7-18.4 mm long (mean = 17.6 mm; n = 5) and 4.1-6.4 mm in diameter (mean = 5.3; n = 5). They were composed of a thin single layer of finely spun silk (0.06 mm thick; measured with a precision s/s calliper), shiny and brownish in colour yet translucent, affixed to the cell walls with numerous silk strands (Fig. 6). The meconium was discharged by the pupating larva internally at the anal end of the cocoon along with the shed integument.
There was no apparent spatial determinism in the location of the parasitised cells. Additionally, both male and female eumenine wasps emerged from the two SLT traps, so there was no apparent host sexual preference by the parasitoid.
Xenorhynchium sp. fashions cell partitions out of fine clay/soil with inclusion of sand grains, approximately 2-3 mm thick; however, Hadrocryptus perforator managed to perforate this obstacle without any apparent difficulty, creating an opening approximately 4 mm in diameter. Although Zethus sp. constructs thin cell partitions probably composed of glandular secretions, the first parasitoid to emerge from trap PSO-117.A6 did so by chewing its way through the trap walls (bamboo), rather than through the cell partitions.
As evidenced by trap NTC-028.A4 and PSO-117.A6, Hadrocryptus lays its egg well after the nest has been closed and sealed by the host. It does so by ovipositing through the trap walls (bamboo) and not by physically entering the nest, as shown by the intact partitions at tube opening. From the very limited data (tube SLT-025.A1 only) it seems that the parasitoid located in the outermost cell emerges first.
There was a 1:1 female to male ratio (n = 8) in the reared specimens.
The emergence of Hadrocryptus perforator was from March to August and its development time is approximately three weeks, therefore it can be inferred that this cryptine is multivoltine in Hong Kong, with the last generation overwintering as prepupal larvae or pupae. In fact the voltinism of this species might coincide with that of its hosts.
Trap NTC-028.A4 at opening.
Trap SLT.025.A1 at opening.
Trap SLT.025.A4 at opening.
The emergence sequence of an adult Hadrocryptus perforator from trap SLT-025.A4.
Cell 4 of trap SLT-025.A4 showing the coloration of the pupa inside the cocoon.
Larva of Hadrocryptus perforator feeding on prepupal larva of Zethus sp., trap PSO-117.A.6.
Habitus of female Hadrocryptus perforator.
Although most host records of gabuniines are from wood-boring Coleoptera, reliable host records are in short supply and parasitism of stem-nesting aculeates may have evolved more frequently, as has been the case in Pimplinae (
We are extremely grateful to Jun-ichi Kojima and Lien Nguyen, Ibaraki University, Japan, who identified the two eumenine hosts, and Graham Reels, Hong Kong who provided structural comments on an early version of the manuscript. Two anonymous reviewers provided detailed and useful reviews.