(C) 2012 Mao-Ling Sheng. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
An enigmatic taxon collected in China is described as Bicurta sinica gen. n., sp. n. The unique specimen proved difficult to place to subfamily but has features in common with Collyria, until recently the sole valid genus of the small Palaearctic subfamily, Collyriinae. A morphological phylogenetic analysis of the pimpliformes group of subfamilies confirms the placement of this genus in the Collyriinae, which is here redefined.
new genus, new species, taxonomy, China, Poemeniinae, pimpliformes
The speciose family Ichneumonidae is currently divided into about 38 subfamilies (
Two of the authors (M-LS and S-PS) received a single specimen of a distinctive ichneumonid collected in Jiangxi province, P.R. China. Unfortunately, further fieldwork by M-LS and S-PS in the type locality has failed to uncover any further specimens. This specimen proved very difficult to place in any subfamily, with a combination of character states that could almost equally feasibly place it in Poemeniinae or Collyriinae. For example, both subfamilies have an elongate propodeum with the spiracle only a little anterior to the middle; they both often lack transverse carinae on the propodeum; the hind coxa is elongate; the first metasomal segment is usually of a similar, elongate shape; the first abscissa of hind wing vein cu is much shorter than vein cu-a. This specimen, which obviously represents an undescribed species and genus, lacks the apomorphies of Collyria, namely the decurved ovipositor with small serrations along the lower margin and the distinctive claw characters (fore and mid tarsal claws each with a median tooth), although it does have lobate fore and mid claws, in common with several pimpliform taxa. The new specimen lacks the principle apomorphies of the Poemeniinae, namely the ventral continuation of the epomia parallel to the lower margin of the pronotum and the laterally expanded foramen magnum (although this is difficult to see in the single new specimen). As well as the character states that are common to Poemeniinae and Collyria, the new specimen shows three characters of the head that led us to believe that its affinities are closer to Collyria, namely the short antennae, the presence of a bifurcate carina from the dorsal half of the face to between the antennae, and a weak median tubercle on the clypeus. After much deliberation, we hypothesised that this specimen represents a new genus of Collyriinae. To test this hypothesis, we coded the specimen of Bicurta sinica sp. n. and Collyria coxator (Villers) for
For the phylogenetic analyses, codings were based on the matrix of
The character codings for Bicurta gen. n. and Collyria are shown in Table 1 with the character numbers as in
Character 92. Dorsal face (0) lacking a bifurcate carina between the antennal sockets (Fig. 6); (1) possessing a bifurcate carina between the antennal sockets.
Character 93. Antenna length (0) normal, at least 0.8× length of fore wing; (1) short, only about 0.65-0.7× length of fore wing.
Both of these characters were coded as ‘0’ for other taxa in
We have also made some changes to the character codings employed by
Phylogenetic analyses were carried out in TNT 1.1 (
SEM images of uncoated specimens were taken using a Leo 1455VP low vacuum scanning electron microscope. Photographs of Bicurta sinica sp. n. were taken using a Canon Power Shot A650 IS and Cool Snap 3CCD attached to a Zeiss Discovery V8 Stereomicroscope and captured with QCapture Pro version 5.1.
Character codings for Bicurta and Collyria; characters 1-91 are from
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| Bicurta | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ? | ? | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | ? |
| Collyria | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | |
| Bicurta | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 |
| Collyria | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 |
| 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 | 55 | 56 | 57 | 58 | 59 | 60 | |
| Bicurta | 0 | 0 | 1 | 0 | 1 | n/a | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Collyria | 0 | 0 | 1 | 0 | 0 | n/a | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 | 71 | 72 | 73 | 74 | 75 | 76 | 77 | 78 | 79 | 80 | |
| Bicurta | 1 | 1 | 0 | 0 | ? | ? | 0 | 0 | 1 | 0 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Collyria | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ? | 1 | 0 | 0 | 2 | 0 | 0 | 0 |
| 81 | 82 | 83 | 84 | 85 | 86 | 87 | 88 | 89 | 90 | 91 | 92 | 93 | ||||||||
| Bicurta | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 1 | |||||||
| Collyria | ? | ? | 1 | 0 | 1 | 0 | 0 | ? | 1 | ? | 0 | 1 | 1 |
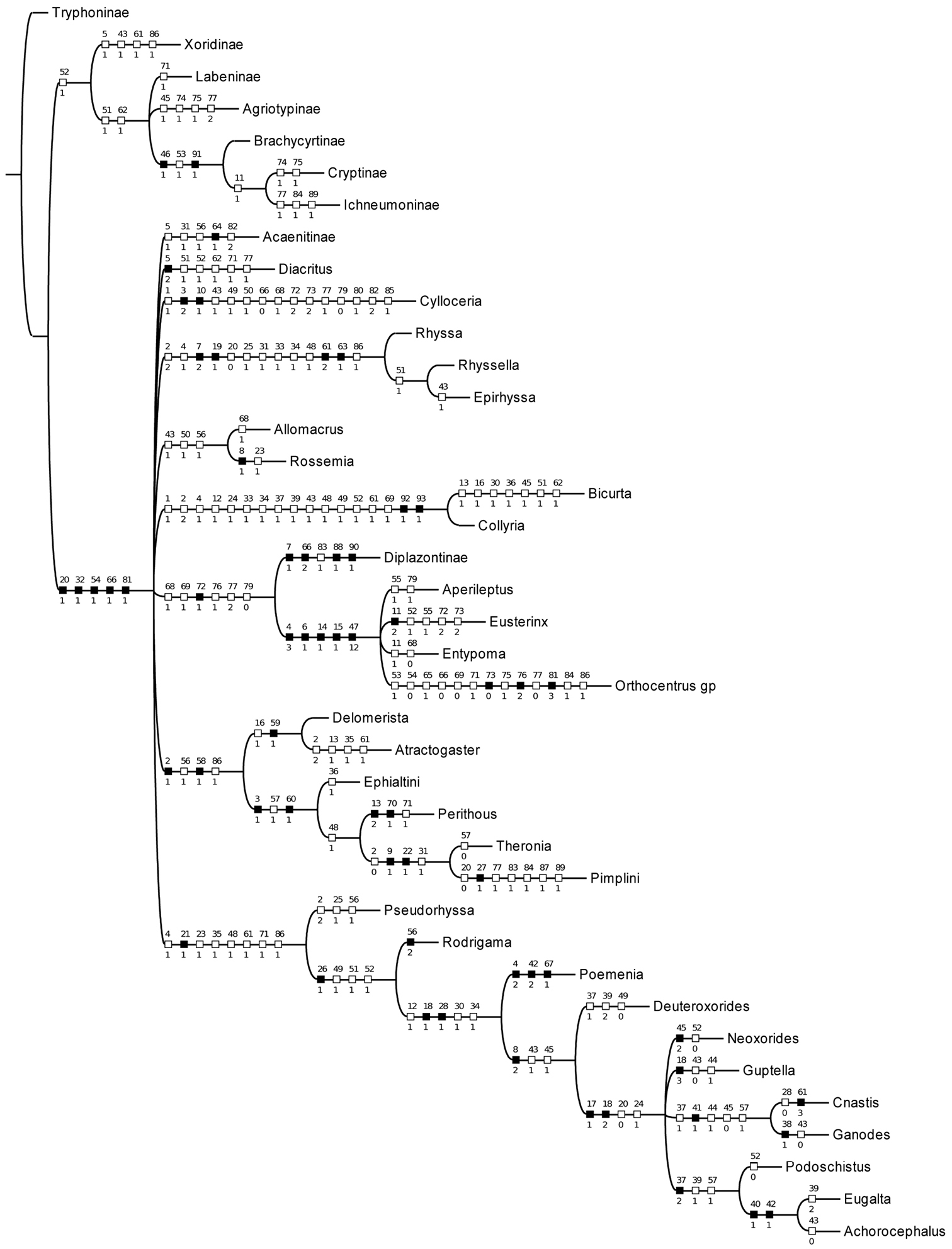
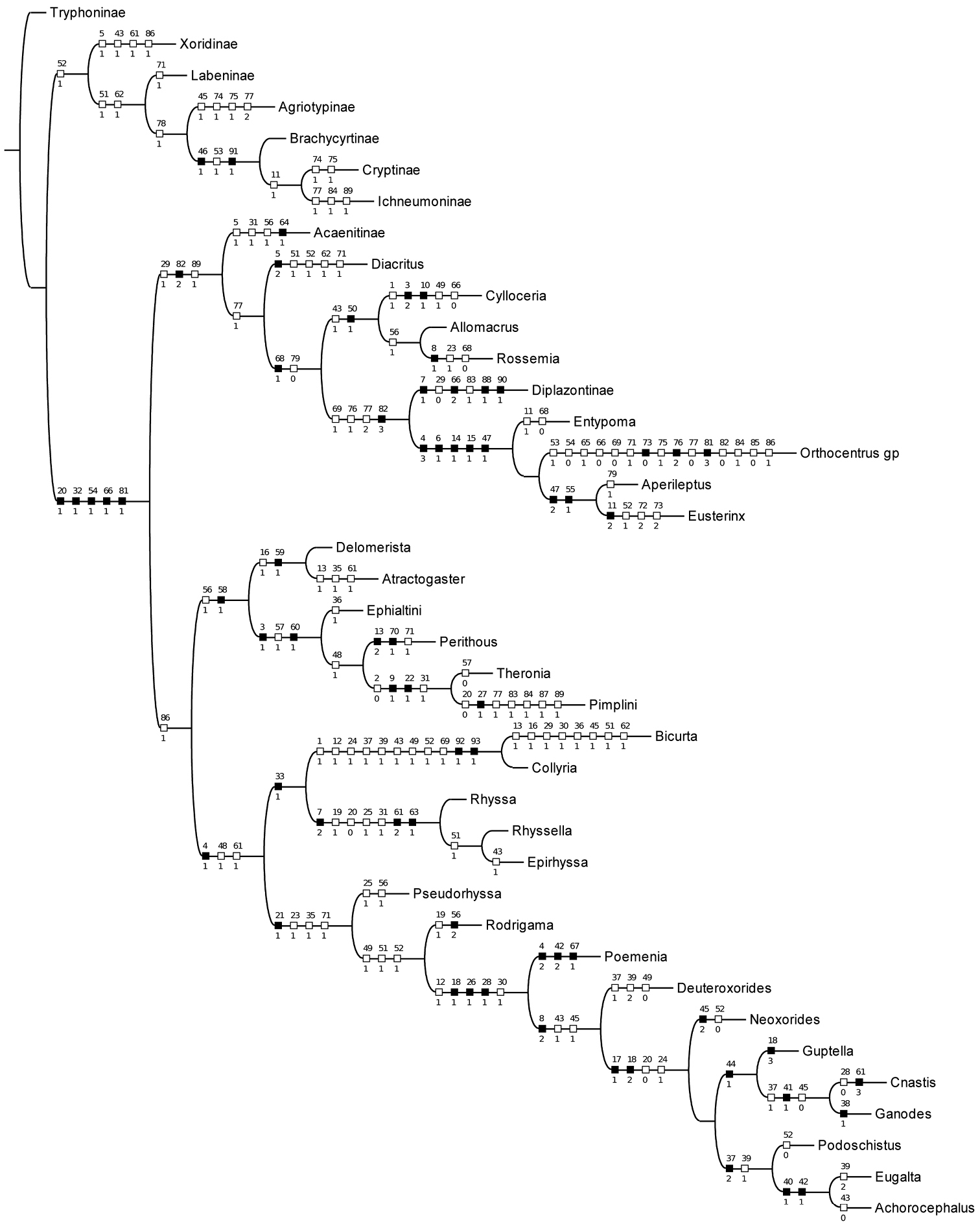
With characters unordered and unweighted, 12 trees of length 240 were found; the strict consensus is shown in Fig. 1. The relationships betweeen subfamilies are largely unresolved but Bicurta and Collyria are recovered as a clade, as are all other subfamilies with multiple representatives, except Cylloceriinae. Searching with implied weights of values ranging from k=1 to k=10 resulted in a single topology, shown in Fig. 2. This phylogeny is similar to that obtained by
In these analyses, the monophyly of Collyriinae is attested by numerous homoplasies and two synapomorphies: the presence of a bifurcate carina on the upper face extending to between the antennal sockets and the short antennae. The sister group relationship of Collyriinae to Rhyssinae was unexpected and is supported by a single apomorphy, the absence of the posterior transverse carina of the propodeum. The monophyly of Poemeniinae+Rhyssinae+Collyriinae is again weakly supported by one unambiguous apomorphy (the small and subrectangular clypeus) and two homoplasies (hind wing vein 2/Cu originating close to vein M and the elongate last visible tergite of the female). In contrast,
Morphological characters and URIs to concepts in the Hymenoptera Anatomy Ontology.
Cladogram of selected pimpliformes taxa. Strict consensus of 12 trees of length 240. Apomorphic characters are indicated by black squares, homoplasies by white squares.
Cladogram of selected pimpliformes taxa obtained by reweighting with implied weights, k=3.
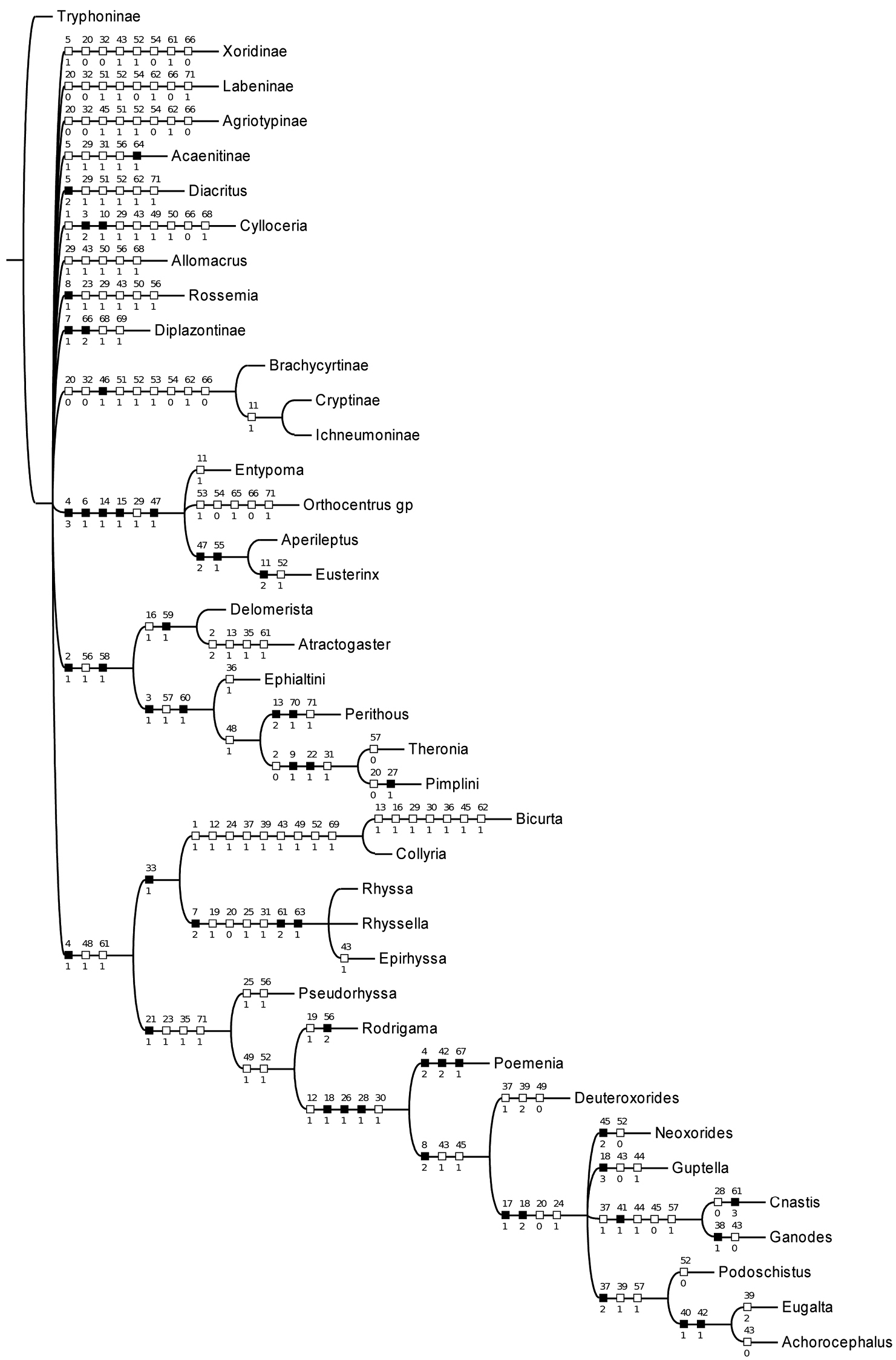
Strict consensus of 89 trees resulting from analysis of reduced data matrix (characters 72–93 excluded).
The Collyriinae, now comprising the genera Aubertiella, Bicurta gen. n. and Collyria, can be defined by the following characters, based on the phylogenetic analyses presented here and on the description of Aubertiella (
An expanded phylogenetic matrix, encompassing the genera of Acaenitinae, could reveal some rather different patterns of relationships within the pimpliformes. Several acaenitine genera share character states with collyriines and some poemeniines, such as short antennae, a median tubercle on the clypeus, hind wing vein Cu1 originating close to M and the propodeum lacking transverse carinae. However, acaenitines have a distinctive apomorphic female hypopygium, very different to that of the Collyriinae, Poemeniinae or Rhyssinae.
TaxonomyCollyriinae can be distinguished from all other subfamilies of Ichneumonidae by the following combination of characters: 1) dorsal part of face with a bifurcate carina extending between the antennal sockets and 2) antenna short, only slightly longer than combined length of head and mesosoma, 0.65–0.7× length of fore wing. Additional distinctive characters, in combination (individually, all are shared with other taxa) are the elongate propodeum with strong lateromedian longitudinal carinae, very stout hind femur, elongate hind coxa and the subclavate shape of the metasoma.
As the concept of Collyriinae has now been expanded since
Antenna short, c. 0.65–0.7× length of fore wing. Male flagellum without tyloids. Mesosoma subcylindric. Occipital carina complete, evenly arched dorsally. Ventrally reaching hypostomal carina well behind base of mandible. Dorsal part of face with a bifurcate carina extending between antennal sockets. Clypeal suture vestigial between clypeal foveae, clypeus faintly convex, apical margin with median tooth or protruberance. Basal portion of mandible wider, strongly narrowed toward apex, teeth sharp, teeth subequal or lower tooth longer than upper tooth. Maxillary palpus with 5 segments, labial palpus with 4 segments. Foramen magnum not expanded laterally. Anterior slope of mid lobe of mesoscutum approximately vertical. Epomia absent. Notaulus long. Epicnemial carina present. Postpectal carina incomplete. Propodeum long, rather cylindrical, longitudinal carinae developed to varying degrees, transverse carinae absent, juxtacoxal carina absent, propodeal spiracle oval. Apex of fore tibia without a tooth on outer side. Fore and hind tibiae each with two spurs. Fore and mid tarsal claws each with either tooth at mid-length or basal lobe, hind tarsal claw large, simple, strongly curved. Hind femur stout, 3.0–3.6× as long as maximally deep. Metasoma subclavate, weakly laterally compressed in distal half. First metasomal segment long, narrow, spiracles anterior to middle, sclerotized part of first sternum extending to middle of tergite or anterior to this. Last visible tergite usually elongate. Hypopygium not elongate. Ovipositor slightly to markedly decurved. Fore wing vein 1cu-a opposite 1/M, vein 3rs-m absent. Hind wing with abscissa of Cu between M+Cu and cu-a strongly reclivous, about 0.2× as long as cu-a.
Collyria coxator (Villers) is a common parasitoid of Cephus pygmaeus (Linnaeus) (Hymenoptera: Cephidae) in Europe and a detailed account of its life history was published by
The nine described Collyria species are found across the Palaearctic, although with few published records from the Eastern Palaearctic (
Aubertiella nigricator (Aubert, 1964) (originally described in Collyria), Collyria catoptron Wahl, 2007; Collyria coxator (Villers, 1789); Collyria distincta Izquierdo & Rey del Castillo, 1985; Collyria fuscipennis (Kriechbaumer, 1894); Collyria iberica Schmiedeknecht, 1908; Collyria isparta Gurbuz & Kolarov, 2006; Collyria orientator Aubert, 1979; Collyria sagitta Kuzin, 1950; Collyria trichophthalma (Thomson, 1877); and Bicurta sinica sp. n.
urn:lsid:zoobank.org:act:82873255-27E4-4CD0-9742-524EED50BF7B
Bicurta sinica Sheng, Broad & Sun, sp. n.
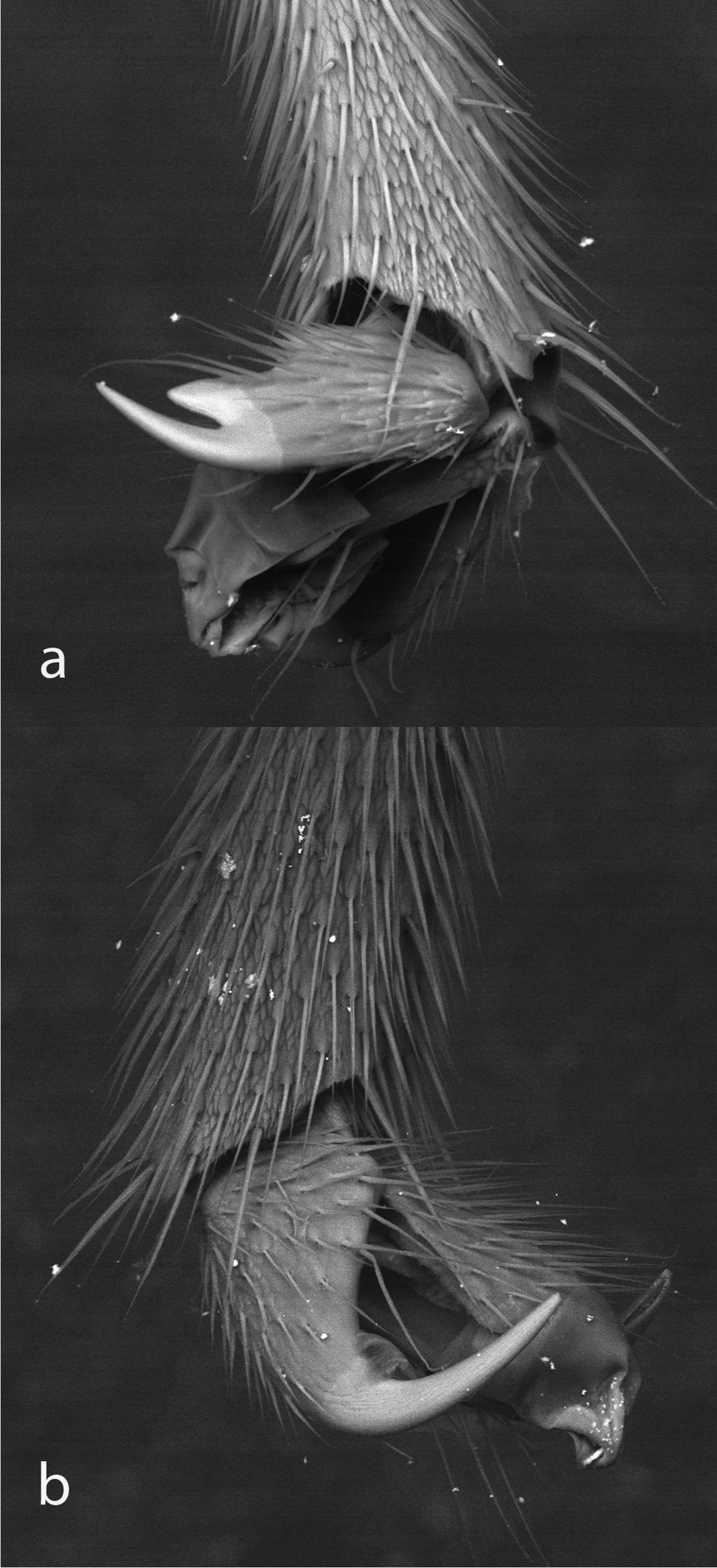
Bicurta can be distinguished from Collyria by any of the following characters (state in Collyria in brackets: 1) epicnemial carina not clearly visible dorsal to mesosternum (carina distinct on mesopleuron); 2) ovipositor straight and smooth, without teeth on ventral valve (weakly decurved with weak teeth on ventral valve in most species; 3) fore and mid tarsal claws with acutely lobed tooth (with a weak medial tooth). Aubertiella resembles Bicurta in the very weak clypeal tubercle and simple ovipositor but can be distinguished by the median teeth on the fore and mid tarsal claws (similar to Collyria), black face and the apical tergites retracted beneath the sixth tergite.
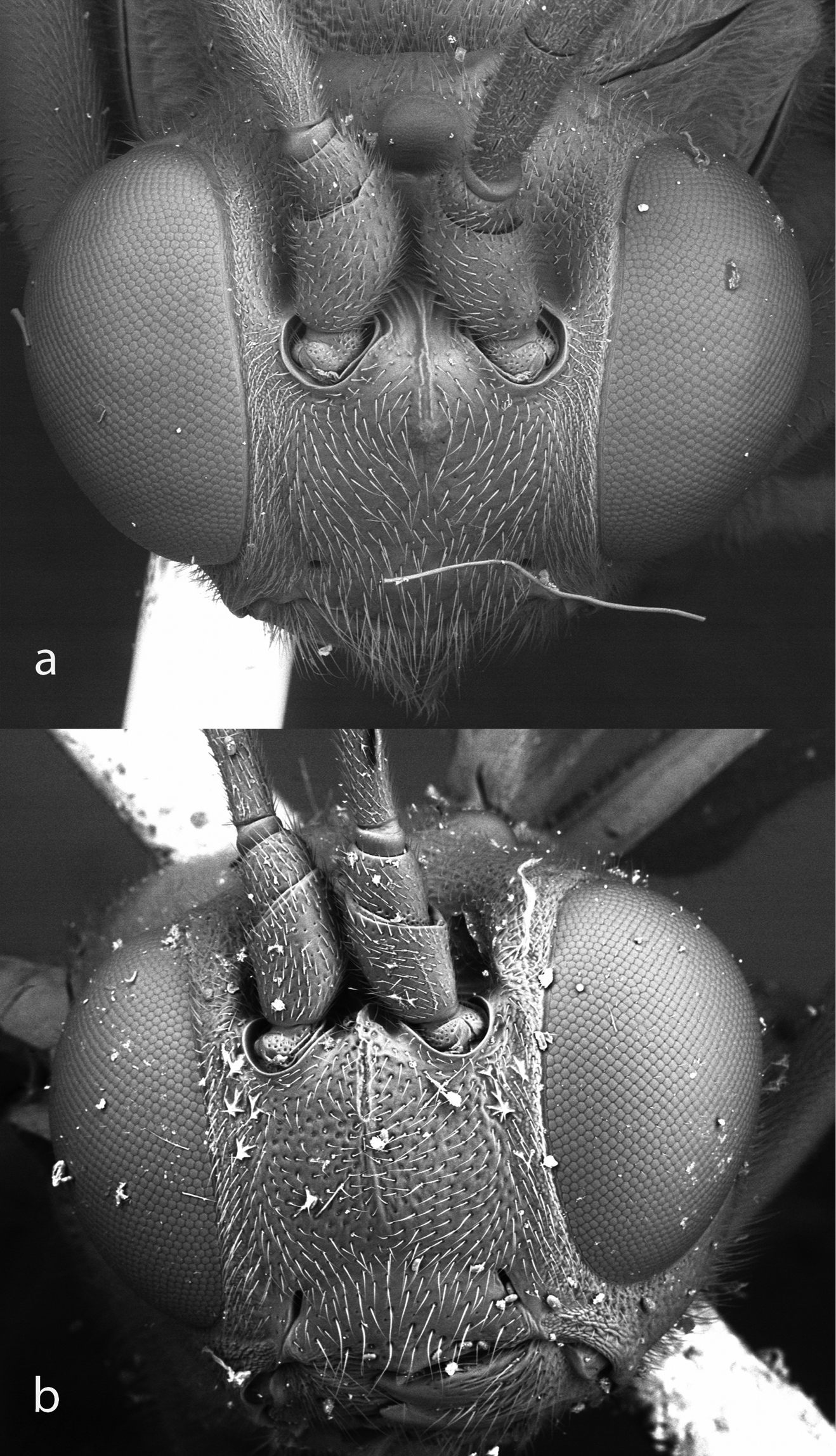
Clypeus nearly flat in lateral view, about 2.2 × as wide as high in anterior view (Fig. 10), median section of apical margin almost truncate, with an obtuse median tubercle or angulation. Mandible strongly narrowed toward apex, teeth sharp, lower tooth slightly longer than upper tooth. Dorsal part of face with bifurcate carina that extends between antennal sockets (Fig. 6a). Antenna short, 0.66x fore wing length, almost clubbed. Notaulus deep, reaching to middle of mesoscutum level with posterior margin of tegula (Fig. 11b). Epicnemial carina indistinct, not clearly visible dorsal to mesosternum (Fig. 11a) although slight furrow can be traced dorsally, far posterior to front edge of mesopleuron. Sternaulus vestigial on anterior 0.4 of mesopleuron. Scutellum and postscutellum approximately flat. Anterior section of submetapleural carina vestigial. Areolet absent. Fore wing with vein 1cu-a opposite 1/M; 2m-cu slightly inclivous, with one bulla. Hind wing with abscissa of Cu between M+Cu and cu-a much longer than cu-a (Cu1 originating close to vein M). Fore and mid tarsal claws elongate with acutely lobed tooth (Fig. 8a). Hind tarsal claw simple (Fig. 8b). Hind coxa elongate, almost as long as first tergite. Hind femur stout, 3.3x as long as maximally deep. Hind leg particularly long, in total 1.9x length of fore wing. Hind coxa elongate, about 0.8 × as long as hind femur, Propodeum elongate, with complete longitudinal carinae, median longitudinal carinae slightly convergent posteriorly, without transverse carinae (Fig. 5a). Propodeal spiracle obliquely elliptical, located at about mid-length of propodeum. Basal portion of metasoma narrow and elongate, apical portion laterally compressed. First tergum approximately 5 × as long as apical width, without longitudinal carinae; sternum reaching half length of tergum, fused with tergum; without glymma; spiracle located at basal 0.42. Ovipositor smooth, without teeth on ventral valve (Fig. 9). Otherwise as in the description of the subfamily.
The name of the new genus is based on the short antenna and ovipositor sheath. The gender is feminine.
urn:lsid:zoobank.org:act:5A7C89FD-5BD7-4574-81F0-4517B3B7FCD6
http://species-id.net/wiki/Bicurta_sinica
Figs 4–11Holotype female, CHINA: Guanshan, 430 m, Yifeng County, Jiangxi Province, 20 April 2009, leg. Ling-Li Yi and Yi Li (GSFPM).
Habitus: Fig. 4. Female. Body length 10.5 mm. Fore wing length 7.6 mm. Ovipositor length about 1.5 mm.
Face approximately flat, 1.4 × as wide as long, with even punctures, distance between punctures 0.2 to 1.0 × diameter of puncture, lateral sides (inner orbit) impunctate and with fine granular texture. Clypeus nearly flat, about 2.2 × as wide as long, with fine, sparse punctures, distance between punctures 2 to 4 × diameter of puncture, apical portion smooth, impunctate. Labrum crescentic, about 0.33 × as long as wide. Malar space with fine leathery texture, without subocular sulcus, approximately 0.4 × as long as basal width of mandible. Gena glossy, with distinct fine punctures, in lateral view approximately 0.66 × as long as width of eye, evenly convergent backward. Posterior portion of vertex with fine punctures, portion between lateral ocellus and eye with fine leathery texture. Interocellar area slightly convex, with fine longitudinal wrinkles. Postero-ocellar line approximately as long as ocular-ocellar line. Dorsolateral part of frons with fine punctures, distance between punctures about as long as diameter of puncture; median portion narrowly smooth longitudinally; ventral portion with weak median longitudinal carina reaching to median protuberance of face. Antenna 5 mm, with 20 flagellomeres, ratio of length of flagellomere 1:2:3:4:5 is 5.5:4.0:3.8:3.7:3.4; last flagellomere 3 × as long as wide, approximately as long as fourth flagellomere. Distance from hypostomal carina to mandible about as long as basal width of mandible.
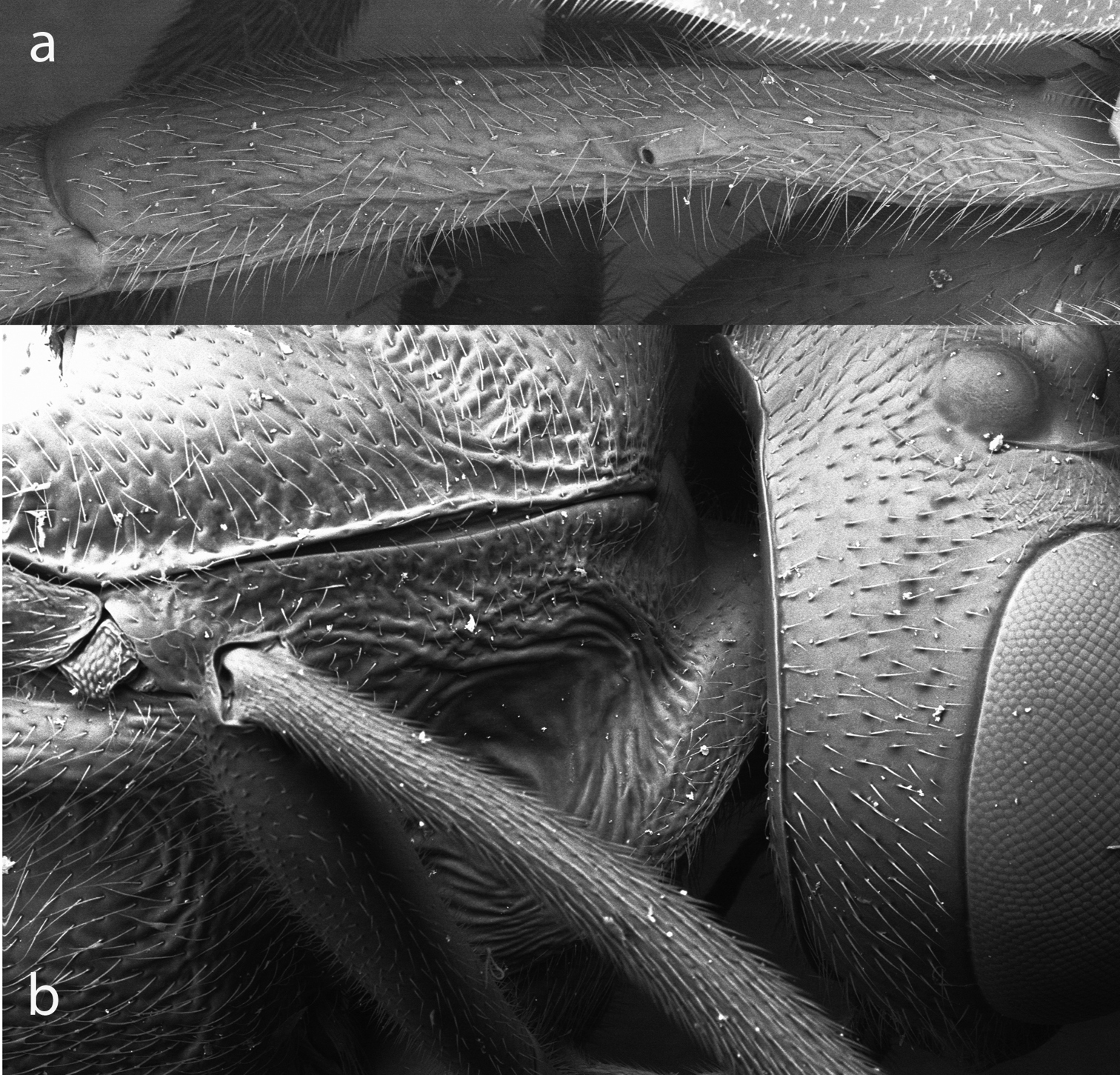
Anterior portion of pronotum with fine longitudinal wrinkles; dorsal portion slightly scabrous; near dorsomedian portion with transverse wrinkles, posterior sections of the wrinkles parallel dorsal margin of pronotum; ventral portion with dense transverse wrinkles. Epomia indistinctly differentiated from strong oblique wrinkles. Mesoscutum with fine punctures; punctures on middle lobe denser than on lateral lobe, distance between punctures 0.2 to 2.5 × diameter of puncture; distance between punctures on lateral lobe 0.5 to 3.5 × diameter of puncture; posterior median portion with longitudinal concave and transverse wrinkles. Scutellum with dense punctures, distance between punctures 0.2 to 0.5 × diameter of puncture. Postscutellum with relatively large, elongate punctures. Lower portion of mesopleuron slightly scabrous, with dense punctures; dorsoanterior portion, in front of subalar prominence, with short longitudinal wrinkles; median portion (anterior to speculum) and ventroposterior portion with short transverse wrinkles. Speculum relatively large. Mesosternum densely punctate. Median longitudinal suture of mesosternum distinct, slightly widening posteriorly. Metapleuron scabrous, with irregular, elongate punctures. Metasternum elongate, approximately 0.6 × as long as mesosternum, with distinct median longitudinal carina and irregular transverse wrinkles. Wing hyaline with slight grey tinge. 2rs-m basad 2m-cu by 0.66 × length of 2rs-m. Vein 2-Cu as long as 2cu-a. Hind coxa with distinct punctures. Ratio of length of hind tarsomeres 1:2:3:4:5 is 10.0:4.2:2.9:2.0:4.2. Propodeum between carinae with distinct transverse wrinkles. Propodeal spiracle 1.4 × as long as maximum width, distance to pleural carina approximately 2.6 × as long as distance to lateral longitudinal carina.
First tergum approximately 5 × as long as apical width, with longitudinal wrinkles, between wrinkles with punctures; without longitudinal carina; spiracle convex, located at basal 0.42. Second tergum about 2.0 × as long as apical width, slightly widened posteriorly, with sparse, indistinct punctures. Third tergum with even, fine hairs, gradually weaker and indistinct posteriorly. Ovipositor sheath about 0.27 × as long as hind tibia. Ovipositor very slightly compressed.
Black. Ventral, inner orbits, clypeus, stripe passing through anterior tentorial pits, mandible except teeth, yellow; ventral profile of antenna brown to yellowish brown. Labial and maxillary palpi, fore and mid legs, trochantellus of hind leg, hind tarsomeres buff. Apex of hind coxa, ventral profile of trochanter of hind leg, basal and apical portion of hind femur brown. Basal 0.65 of hind tibia dull yellow, fading to dark brown apical 0.35. Tegula dark brown. Hind margins of terga 3 to 6 narrowly yellow. Wing venation, including pterostigma dark brown.
Named after the country, China, where the unique specimen was collected.
A distinctive species with the short, featureless ovipositor, rather massive hind leg, lobate fore and mid claws, short antennae and well-marked facial pattern.
Unfortunately, nothing is known of the biology of Bicurta sinica sp. n. Where known, species of Collyria are koinobiont egg-larval endoparasitoids of stem-sawflies (Hymenoptera: Cephidae) (
An expanded phylogenetic matrix, encompassing the genera of Acaenitinae, could reveal some rather different patterns of relationships within the pimpliformes. Several acaenitine genera share character states with collyriines and some poemeniines, such as short antennae, a median tubercle on the clypeus, hind wing vein Cu1 originating close to M and the propodeum lacking transverse carinae, and it is interesting that various older authors included Collyria within the Acaenitinae (or equivalent grouping). Obviously, acaenitines have a distinctively apomorphic female hypopygium, very different to that of the Collyriinae, Poemeniinae or Rhyssinae.
Bicurta sinica Sheng, Broad & Sun, sp. n., habitus.
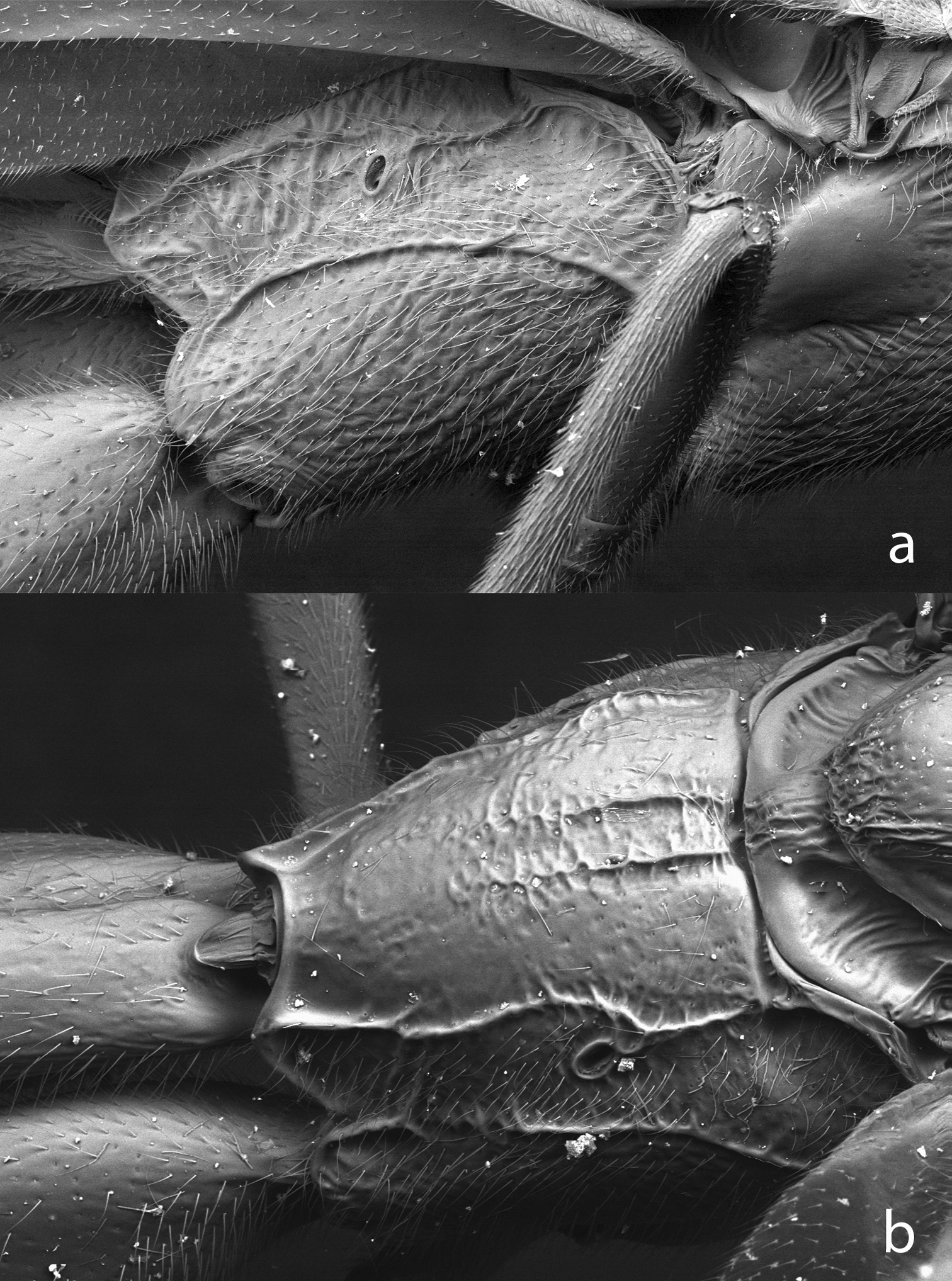
Propodeum of Bicurta sinica a and Collyria coxator b. Figs 5–9, SEMs of uncoated specimens.
. Head, frontal view, of Bicurta sinica a and Collyria coxator b.
First tergite a and pronotum b of Bicurta sinica.
Fore claw a and hind claw b of Bicurta sinica.
Ovipositor of Bicurta sinica.
Face of Bicurta sinica.
Mesopleuron a and mesoscutum b of Bicurta sinica.
We are grateful to Ling-Li Yi and Yi Li for their help in the course of exploration in Jiangxi Province. István Mikó (North Carolina State University) was very helpful in ‘ontologising’ the manuscript, providing the URIs in Table 2, suggesting the concepts that our terms referred to and highlighting various errors we had made. Andrew Bennett and an anonymous referee provided very useful criticism of the manuscript. This research was supported by the National Natural Science Foundation of China (NSFC, No. 30872035; No. 31110103062).