(C) 2012 Keith R. Hopper. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The Aphelinus mali complex consists of eleven described species. Monophyly of this complex is well supported by a combination of traits: (1) a single complete row of setae proximal to the linea calva of the fore wing, with a few additional setae in the angle between this row and the marginal vein; (2) linea calva open (no setae at its posterior edge); (3) head and body dark except for parts of the metasoma; (4) meso- and metacoxae dark; (5) metafemur pale, (6) metatibia dark. Species within the complex have been distinguished by color and shape of antennal segments (particularly the third funicular segment), color of legs and metasoma, and relative length of ovipositor versus mesotibia. We provide a key for identifying species in the mali complex, and describe three new species, Aphelinus glycinis sp. n., Aphelinus rhamni sp. n., and Aphelinus coreae sp. n. from material in laboratory cultures originally reared from soybean aphid in China and Korea as candidates for biological control of soybean aphid, Aphis glycines.
cryptic species, taxonomy, biological control
The genus Aphelinus (Hymenoptera: Aphelinidae) comprises 84 recognized species (
The genus Aphelinus consists of several complexes of cryptic species including the mali complex, the varipes complex (
Species in Aphelinus mali complex and related species
| Group | Species | Original description |
| mali complex | Aphelinus basilicus |
|
| Aphelinus campestris |
|
|
| Aphelinus coreae sp. n.1 | ||
| Aphelinus engaeus |
|
|
| Aphelinus ficusae |
|
|
| Aphelinus glycinis sp. n.1 | ||
| Aphelinus gossypii |
|
|
| Aphelinus mali |
|
|
| Aphelinus niger2 |
|
|
| Aphelinus paramali |
|
|
| Aphelinus rhamni sp. n.1 | ||
| Aphelinus sanborniae |
|
|
| Aphelinus siphonophorae2 |
|
|
| Aphelinus spiraecolae |
|
|
| related species | Aphelinus chaonia3 |
|
| Aphelinus prociphili3 |
|
|
| Aphelinus sharpae3 |
|
|
| Aphelinus brunneus4 |
|
|
| Aphelinus daucicola4 |
|
|
| Aphelinus lapisligni4 |
|
1 new species described in this paper 2 insufficient description to be included in tree or key 3 difference from mali complex: more than 1 line of setae in delta region 4 difference from mali complex: posterior femur dark
Three new species in the Aphelinus mali complex were collected from Aphis glycines in the Peoples Republic of China near Beijing and Xiuyan (Liaoning Province) and in the Republic of South Korea near Miryang (Gyeongsangnam Province) and maintained as laboratory cultures at the Beneficial Insects Introduction Research Unit, USDA-ARS, Newark, DE. All of the specimens described below were taken from lab cultures, killed in 95% ethanol, and most were critical-point-dried and card-mounted. Selected specimens were then slide-mounted in Canada balsam. Specimens photographed for coloration (Figs 1–6, 15–20, and 29–34) were killed in ethanol and photographed as soon as possible, by placing specimens on a layer of KY® jelly in a small watch glass, submerging the specimen in ethanol, and photographing using a Leica MZ 16 stereomicroscope, fiber optic illumination, a Zeiss Axiomat MRc5 camera, and Helicon Pro image-stacking software. Slide-mounted specimens were photographed using differential interference contrast optics (DIC) with an Olympus BH2 compound microscope, and the same camera and software. Final modifications to images were made using Adobe Photoshop, Adobe Lightroom, and Adobe InDesign. Type material and other specimens examined have been deposited as indicated in the species descriptions. The label data for each specimen has been digitized and all specimens bear individual accession numbers for Texas A&M University Insect Collection (e.g. TAMU x0616203), as well as a machine-readable bar-code. In the verbatim label data provided for holotypes, a single | symbol indicates a new line on a label, and the || symbol indicates a second or third label. Vouchers are maintained at -20oC in molecular grade ethanol at the Beneficial Insect Introduction Research Unit, Newark, Delaware, and at the Department of Entomology, Texas A&M University, College Station, Texas.
We tabulated and coded 19 traits for species in the Aphelinus mali complex, using the original species descriptions for the most part. These traits included color of scape, pedicel, club, coxae, femora, tibiae, tarsi, and metasoma, as well as shape of third funicle and club (length:width) and length of ovipositor relative to mesotibia. For some traits, males and females differed (e.g., F3 shape, procoxae color) and the values were scored separately. When trait data were lacking from original descriptions, we used data from later descriptions. Trait values for the new species in the complex were taken from specimens freshly killed in ethanol and slide-mounted specimens. These traits were used to construct an on-line, interactive, multiple entry identification key to the mali complex which is available on request. Of the 19 traits, 12 proved to be most consistent and useful in distinguishing species, and these are presented in Table 2.
Table 3 is a list of anatomical terms used in the paper followed by URI values (uniform resource identifiers), that will link the terms to precise definitions and illustrations in the Hymenoptera Anatomy Ontology project (see http://portal.hymao.org and http://hymao.org for more information on this initiative). Additional information on morphological terminology in Chalcidoidea is available in
The ventral surface of the antennal scape refers to the surface that is ventral when the antennae are deployed, or anterior when the antennae are folded on the face. F1, F2 and F3 refer to the first, second and third segments of the funicle of the antennal flagellum, respectively. T1, T2 etc. refer to metasomal terga. We use the term ovipositor to refer to the anatomical cluster consisting of the first valvula, second valvula, third valvula, first valvifer and second valvifer. Length of the ovipositor is the measurement (generally of a slide-mounted specimen) from the anterior margin of the second valvifer to the posterior (distal) end of the third valvula.
Measurements were made with an eyepiece reticle in a Leica MZ16 microscope or Zeiss standard 16 compound microscope, or from digital images captured using the methods described above. As with any species of Aphelinus, users will require series of high quality specimens, both male and female, and both card- or point-mounted and slide-mounted specimens, to obtain confident identifications.
Traits coded for species in mali complex of Aphelinus.
| Species | F3 female1 | F3 male2 | Club female3 | Club male4 | Procoxa color5 | Profemur color6 | Mesofemur color7 | Protibia color8 | Mesotibia color9 | Metatibia color10 | Metasoma color11 | Ovipositor to mesotibia12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aphelinus basilicus | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| Aphelinus campestris | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 2 | ? |
| Aphelinus coreae sp. n. | 1 | 1 | 2 | 2 | 1 | 4 | 1 | 3 | 5 | 3 | 2 | 2 |
| Aphelinus engaeus | 1 | 2 | 2 | 2 | 2 | 3 | 4 | 2 | 4 | 3 | 5 | 2 |
| Aphelinus ficusae | 2 | 3 | 2 | 2 | 2 | 3 | 3 | 2 | 3 | 3 | 1 | 1 |
| Aphelinus glycinis sp. n. | 2 | 3 | 2 | 2 | 3 | 3 | 4 | 3 | 2 | 3 | 6 | 2 |
| Aphelinus gossypii | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 2 |
| Aphelinus mali | 1 | 2 | 2 | 2 | 1 | 2 | 5 | 2 | 1 | 1 | 1 | 3 |
| Aphelinus paramali | 2 | 2 | 2 | 2 | 1 | 5 | 2 | 2 | 2 | 2 | 3 | 1 |
| Aphelinus rhamni sp. n. | 1 | 1 | 2 | 2 | 1 | 2 | 1 | 1 | 2 | 3 | 2 | 2 |
| Aphelinus sanborniae | 2 | 3 | ? | ? | 1 | 1 | 1 | 4 | 2 | 1 | 4 | ? |
| Aphelinus spiraecolae | 1 | 1 | 1 | 1 | 1 | 3 | 4 | 2 | 4 | 1 | 1 | 3 |
1- F3 female: 1 = subquadrate, 2 = at least 1.2× longer than wide
2- F3 male: 1 = subquadrate, 2 = 1.2 to 2.0× longer than wide, 3 = more than 2.0× longer than wide
3- Club female: 1 = less than 2.5× as long as wide, 2 = at least 2.5× as long as wide
4- Club male: 1 = less than 3× as long as wide, 2 = at least 3× as long as wide
5- Procoxae: 1 = dark, 2 = yellow, 3 = pale in females, dark grey in males
6- Profemora: 1 = dark, 2 = dark with apex pale, 3 = yellow or pale, 4 = yellow in females, middle part fuscous in males
7- Mesofemora: 1 = dark, 2 = dark with apex pale, 3 = yellowish white, 4 = yellow in females, middle part fuscous in males, 5 = middle part dark brown in both sexes
8- Protibia: 1 = pale brownish yellow with basal half dark brown, 2 = white, yellow or pale, 3 = pale to grey, 4 = pale yellow, often with middle part darker
9- Mesotibia: 1 = black with apex brownish yellow, 2 =- middle part dark, base and apex lighter, 3 = yellow or yellowish white, 4 = pale yellow, middle part dark, 5 = dark with distal half pale
10- Metatibia: 1 = dark brown or black, 2 = dark with apex pale, 3 = black or brown with base white, 4 = pale yellow, often with middle part darker
11- Metasoma: 1 = T1 or T1&T2 yellow, 2 = T1 or T1&T2 and apex pale, 3 = T1-T3 yellow, 4 = dark, black, 5 = female yellow with brown dorsum, except T1&T2, male dark with pale base, 6 = female yellow with brown dorsum except T1&T2, male base and apex pale
12- Ovipositor / mesotibia: 1 = less than 1.2, 2 = 1.3–1.5, 3 = more than 1.5
List of anatomical terms and links to URI locations in the Hymenoptera Anatomy Ontology portal.
| Term | Definition | URI |
|---|---|---|
| antenna | The anatomical cluster that is composed of the scape, pedicel and flagellum. | http://purl.obolibrary.org/obo/HAO_0000101 |
| apical denticle | The spur that is located distally on the gonossiculus. | http://purl.obolibrary.org/obo/HAO_0001574 |
| base | The tergum that is located on abdominal segment 2 AND The tergum that is located on the abdominal segment 3. | http://purl.obolibrary.org/obo/HAO_0000053 and http://purl.obolibrary.org/obo/HAO_0000056 |
| body | The anatomical cluster that is composed of the whole organism but which excludes the antennae, legs and wings. | http://purl.obolibrary.org/obo/HAO_0000182 |
| club | The anatomical cluster composed of the apical flagellomeres that are differentiated by size from the basal flagellomeres. | http://purl.obolibrary.org/obo/HAO_0001185 |
| compound eye | The compound organ that is composed of ommatidia. | http://purl.obolibrary.org/obo/HAO_0000217 |
| costal cell | The membranous region of the forewing anterior to the submarginal vein, measured from the basal constriction that delimits the apex of the humeral plate of the wing to the point at which the submarginal vein touches the leading edge of the wing. | http://purl.obolibrary.org/obo/HAO_0000226 |
| coxa | The leg segment that is connected to the body and to the trochanter via conjunctivae and muscles. | http://purl.obolibrary.org/obo/HAO_0000228 |
| digitus | The sclerite that is located distally on the parossiculus. | http://purl.obolibrary.org/obo/HAO_0000385 |
| edge | The margin that extends along the border of two areas that are oriented differently. | http://purl.obolibrary.org/obo/HAO_0000285 |
| eye margin | The margin of the compound eye. | http://purl.obolibrary.org/obo/HAO_0000672 |
| F1 | The flagellomere that is proximally attached to the pedicel. | http://purl.obolibrary.org/obo/HAO_0001148 |
| F2 | The flagellomere that is located distal to the first flagellomere. | http://purl.obolibrary.org/obo/HAO_0001883 |
| F3 | The flagellomere that is located immediately distal to the second flagellomere. | http://purl.obolibrary.org/obo/HAO_0001895 |
| femur | The leg segment that is distal to the trochanter and proximal to the tibia. | http://purl.obolibrary.org/obo/HAO_0000327 |
| fore wing | The wing that is located on the mesothorax. | http://purl.obolibrary.org/obo/HAO_0000351 |
| frontovertex | The anatomical cluster that is composed of the vertex and the dorsal area of the upper face dorsal to the frontofacial ridge. | http://purl.obolibrary.org/obo/HAO_0001823 |
| genitalia | The anatomical cluster that is composed of the cupula, gonostyle, volsella and the aedeagus. | http://purl.obolibrary.org/obo/HAO_0000312 |
| head | The tagma that is located anterior to the thorax. | http://purl.obolibrary.org/obo/HAO_0000397 |
| hind wing | The wing that is located on the metathorax. | http://purl.obolibrary.org/obo/HAO_0000400 |
| leg | The anatomical cluster that is composed of the coxa and all distal leg segments and is connected to the pectus. | http://purl.obolibrary.org/obo/HAO_0000494 |
| longitudinal sensillum | The multiporous plate sensillum that is elongate. | http://purl.obolibrary.org/obo/HAO_0001936 |
| mandible | The sclerite that is connected to the cranium along the anterior margin of the oral foramen via the anterior and posterior cranio-mandibular articulations. | http://purl.obolibrary.org/obo/HAO_0000506 |
| margin | The line that delimits the periphery of an area. | http://purl.obolibrary.org/obo/HAO_0000510 |
| marginal vein | The abscissa that is located along the anterior margin of the fore wing and is thought to correspond to the anterior abscissa of the radius (R1). | http://purl.obolibrary.org/obo/HAO_0000512 |
| mesobasitarsus | The basitarsus that is located in the mid leg. | http://purl.obolibrary.org/obo/HAO_0001133 |
| mesocoxa | The coxa that is located on the mid leg. | http://purl.obolibrary.org/obo/HAO_0000635 |
| mesofemur | The femur that is located on the mid leg. | http://purl.obolibrary.org/obo/HAO_0001131 |
| mesoscutum | The area that is located anterior to the transscutal articulation. | http://purl.obolibrary.org/obo/HAO_0001490 |
| mesosoma | The anatomical cluster that is composed of the prothorax, mesothorax and the metapectal-propodeal complex. | http://purl.obolibrary.org/obo/HAO_0000576 |
| mesotibia | The tibia that is located on the mid leg. | http://purl.obolibrary.org/obo/HAO_0001351 |
| mesotibial spur | The tibial spur that is located on the mesotibia. | http://purl.obolibrary.org/obo/HAO_0001120 |
| metabasitarsus | The basitarsus that is located on the hind leg. | http://purl.obolibrary.org/obo/HAO_0001142 |
| metabasitarsus | The basitarsus that is located on the hind leg. | http://purl.obolibrary.org/obo/HAO_0001142 |
| metacoxa | The coxa that is located on the hind leg. | http://purl.obolibrary.org/obo/HAO_0000587 |
| metasoma | The tagma that is connected anteriorly to the metapectal-propodeal complex at the propodeal foramen and consists of abdominal segments. | http://purl.obolibrary.org/obo/HAO_0000626 |
| metatibia | The tibia that is located on the hind leg. | http://purl.obolibrary.org/obo/HAO_0000631 |
| metatibial spur | The tibial spur that is located on the metatibia. | http://purl.obolibrary.org/obo/HAO_0001121 |
| mid lobe of mesoscutum | The area that is located between the notauli. | http://purl.obolibrary.org/obo/HAO_0000520 |
| occipital margin | The edge that separates the occiput from the vertex. | http://purl.obolibrary.org/obo/HAO_0001963 |
| ocellus | The multi-tissue structure that is located on the top of the head, composed of the corneal lens, pigment cell, rhabdoms and synaptic plexus. | http://purl.obolibrary.org/obo/HAO_0000661 |
| ovipositor | The anatomical cluster that is composed of the first valvulae, second valvulae, third valvulae, first valvifers and second valvifers . | http://purl.obolibrary.org/obo/HAO_0000679 |
| pedicel | The antennal segment that is the second segment of the antenna and is connected proximally with the scape and distally with the flagellum. | http://purl.obolibrary.org/obo/HAO_0000706 |
| phallobase | The anatomical cluster that is composed of the cupulae, gonostipites and volsellae. | http://purl.obolibrary.org/obo/HAO_0000713 |
| posterior ocellus | The ocellus that is paired. | http://purl.obolibrary.org/obo/HAO_0000481 |
| procoxa | The coxa that is located on the fore leg. | http://purl.obolibrary.org/obo/HAO_0001122 |
| profemur | The femur that is located on the fore leg. | http://purl.obolibrary.org/obo/HAO_0001124 |
| protibia | The tibia that is located on the fore leg. | http://purl.obolibrary.org/obo/HAO_0000350 |
| row | The anatomical cluster that is composed of repeated units of anatomical structures. | http://purl.obolibrary.org/obo/HAO_0000901 |
| scape | The antennal segment that is proximal to the pedicel and is connected with the head via the radicle. | http://purl.obolibrary.org/obo/HAO_0000908 |
| sculpture | The area that is located on the sclerite and that is composed of repetitive anatomical structures. | http://purl.obolibrary.org/obo/HAO_0000913 |
| scutellar sensillum | The campaniform sensillum that is paired and is located submedially on the mesoscutellum. | http://purl.obolibrary.org/obo/HAO_0001965 |
| scutellum | The area that is located posteriorly of the transscutal line and is composed of the axillae and the mesoscutellum. | http://purl.obolibrary.org/obo/HAO_0000572 |
| secretory pore | The anatomical space that corresponds to the distal end of an exocrine gland. | http://purl.obolibrary.org/obo/HAO_0001966 |
| seta | The sensillum that is multicellular and consists of trichogen, tormogen, and sense cells. | http://purl.obolibrary.org/obo/HAO_0000935 |
| side lobe | The area that is located between the notaulus and the parascutal carina. | http://purl.obolibrary.org/obo/HAO_0000466 |
| stigma | The patch on the wing that is sclerotized and is located on the anterior margin of the fore wing. | http://purl.obolibrary.org/obo/HAO_0000957 |
| submarginal vein | Basal-most portion of the forewing vein complex that occurs behind the costal cell; measured from the constriction that delimits the humeral plate to the point at which the vein touches the leading edge of the wing apically. | http://purl.obolibrary.org/obo/HAO_0000972 |
| T1 | The tergum that is located on abdominal segment 2. | http://purl.obolibrary.org/obo/HAO_0000053 |
| T2 | The tergum that is located on the abdominal segment 3. | http://purl.obolibrary.org/obo/HAO_0000056 |
| tarsus | The leg segment that is apical to the tibia. | http://purl.obolibrary.org/obo/HAO_0000992 |
| third valvula | The sclerite that is located posterior to the second valvifer and is connected to the second valvifer via conjuntiva. | http://purl.obolibrary.org/obo/HAO_0001012 |
| tooth | The projection that is located distally on the mandible. | http://purl.obolibrary.org/obo/HAO_0001019 |
| wing | The wing that is located on the mesothorax. | http://purl.obolibrary.org/obo/HAO_0000351 |
Following the work of
| 1 | Female: procoxa white or yellowish-white, male: procoxa yellowish-white or grey, both sexes: meso- and metacoxae dark | 2 |
| – | All coxae dark in both sexes | 4 |
| 2(1) | F3 more than twice as long as broad in male, subquadrate in female | Aphelinus engaeus |
| – | F3 less than twice as long as broad in male and from subquadrate to more than 1.4× as long as broad in female. | 3 |
| 3(2) | Procoxa yellowish white in male; metatibia yellowish white; metabasitarsus pale; club light brown in male; metasoma dark with base yellow in female and slightly pale in male; ovipositor less than 1.2× middle tibia . | Aphelinus ficusae |
| – | Procoxa grey in male, particularly on anterior surface; metatibia dark in center; metabasitarsus greyish brown; club yellow in male; metasoma dark with T1&2, venter, and apex yellow in female and base and apex yellow in male; ovipositor more than 1.2× mesotibia. | Aphelinus glycinis sp. n. |
| 4(1) | F3 longer than broad in male and subquadrate to longer than broad in female; metasoma dark or dark with pale base, but with apex dark | 5 |
| – | F3 subquadrate in both male and female; metasoma dark with pale base or pale base and apex. | 8 |
| 5(4) | Pro- and metafemur dark; protibia dark or dark with yellow apex; metasoma dark or dark with pale base | 6 |
| – | Pro- and metafemur partly yellow; protibia yellow; metasoma dark with pale base. | 7 |
| 6(5) | Scape yellow to pale brown with apical third yellow; pedicel and club infuscate brown; metabasitarsus dark; F3 subquadrate in female and 1.2–2× as long as broad in male; metasoma dark with pale base | Aphelinus basilicus |
| – | Scape dark brown to black; pedicel yellow in female and yellow to dusky in male; club yellow; metabasitarsus yellow; F3 longer than broad in female and more than 2× as long as broad in male; metasoma dark. | Aphelinus sanborniae |
| 7(6) | Pro- and mesofemur dark with base and apex pale; protibia dark with pale base and apex, metatibia dark with pale apex; F3 1.2–1.5 as long as broad in female and 1.2–2× as long as broad in males; ovipositor equal to metatibia; metasoma dark with T1–T3 yellow | Aphelinus paramali |
| – | Pro- and mesofemur dark with apex pale; protibia dark with brownish yellow apex, metatibia dark; F3 subquadrate in female and 1.2–2× as long as broad in male; ovipositor more than 1.5× mesotibia; metasoma dark with T1 or T1&2 yellow. | Aphelinus mali |
| 8(4) | Profemur pale yellow, mesofemur pale yellow in female, dark brown in male; metabasitarsus yellow; metasoma dark with base yellow | Aphelinus spiraecolae |
| – | Profemur dark with apex pale yellow, mesofemur dark in both sexes; metabasitarsus dark; metasoma dark with base and apex yellow. | 9 |
| 9(8) | Club more than 2× as long as broad in female; metatibia dark with pale base | 11 |
| – | Club 2× as long as broad in females; metatibia all dark. | 10 |
| 10(9) | Club and pedicel light brown in female and darker in males; mesofemur dark; mesotibia dark with pale base and apex. | Aphelinus campestris |
| – | Club pale yellow in female and male; pedicel dusky yellow in female and light brown in male; mesotibia dark with brownish yellow apex | Aphelinus gossypii |
| 11(9) | Club more than 3× as long as broad in males; scape yellowish white in female and infuscate brown in male; mesotibia dark with base and apex pale | Aphelinus rhamni sp. n. |
| – | Club less than 3× as long as broad in males; scape dark brown with distal half yellow in both sexes; mesotibia dark with distal half yellow | Aphelinus coreae sp. n. |
urn:lsid:zoobank.org:act:1132B1E4-8F2E-4FF3-9E6B-30FAFD497EA1
http://species-id.net/wiki/Aphelinus_glycinis
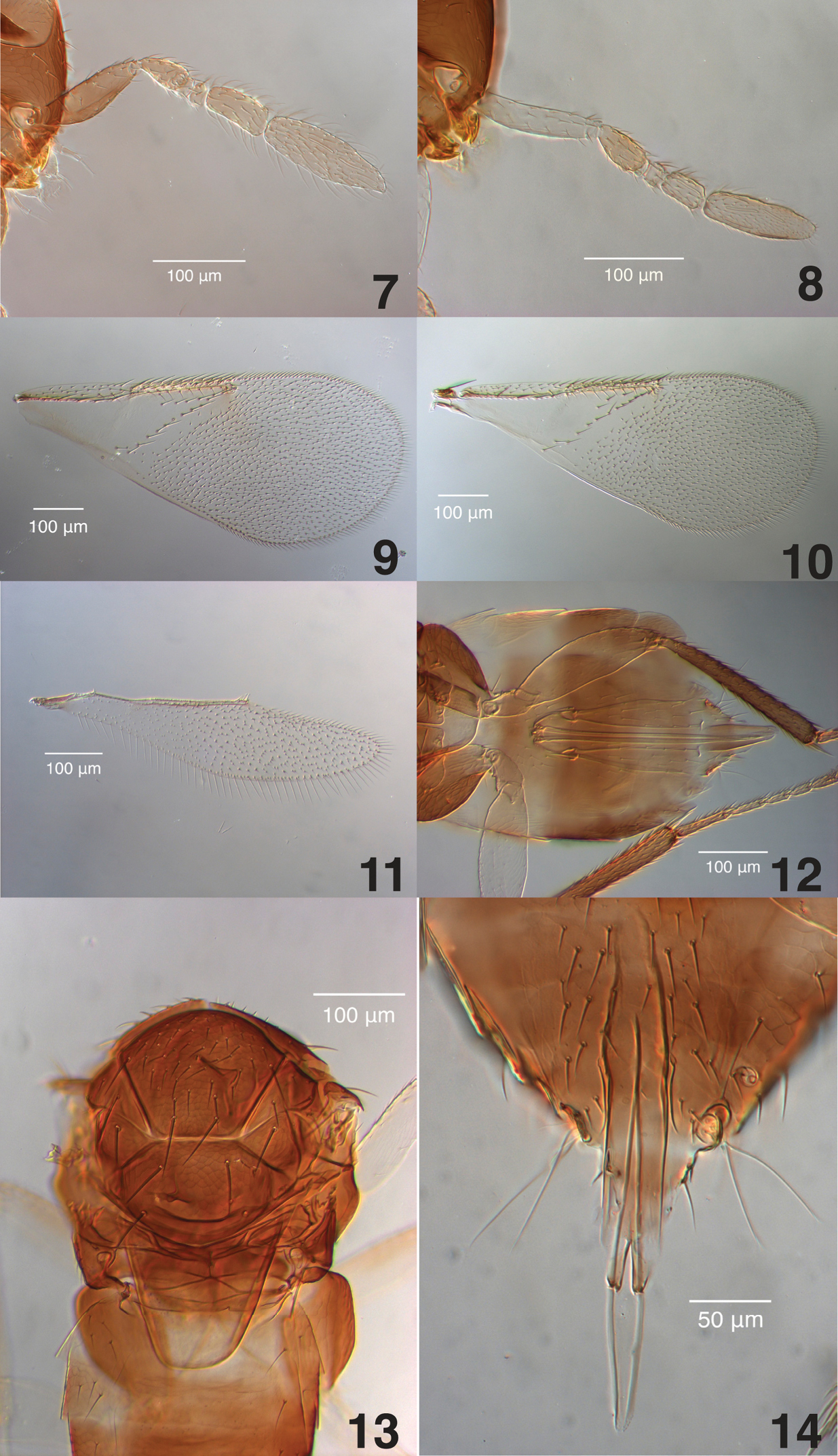
Figs 1- 14Female. Head and mesosoma dark brown to black; legs with procoxa yellowish white, meso- and metacoxae dark brown to black, femora yellowish white, protibia yellowish white, mesotibia yellowish white with center greyish, metatibia dark grey to black with base pale; metasoma with base, apex, and venter yellow, remainder brown; antenna white to yellowish white; F3 1.3–1.7 times as long as broad; club 3.2 times as long as broad. Male similar except procoxa grey; pro- and mesofemur sometimes with darkened center; metasoma brown with base and apex yellow; scape dark greyish brown with greyish yellow distal tip, swollen in center, maximum width 3× distal end width, with three to five volcano-shaped secretory pores in a single line on ventral surface, pedicel greyish yellow, third funicle more than 2 times as long as broad, club 3.9 times as long as broad.
Female (Figs 2, 4, 6, 8, 10, 11, 12, 13).
Body length. 0.77–0.93 (Holotype 0.90 mm).
Head. (Figs 2, 8) Head 1.3× as broad as high in frontal view, about as broad as mesosoma; frontovertex width 0.4× of head width, 1.2× as long as broad, and 0.8× as broad as scape length; posterior ocelli 1.0× their diameter from eye margin, 3× their diameter from one another, and 0.33× their diameter from occipital margin; mandible with 2 acute teeth and a broad truncate surface below the teeth, antenna as in Fig. 8 with scape 4.8× as long as broad, pedicel 2.2× as long as broad, F1 anneliform, 1.7× as wide as long, F2 1.2× as broad as long, F3 1.6–1.7× as long as broad, club 3.2× as long as broad, 2.7× times as long as F3, and with 6–7 longitudinal sensilla.
Mesosoma. (Figs 4, 6, 13) Mesoscutum and scutellum with fine reticulate sculpture, longest diameter of reticulations approximately twice the diameter of the scutellar sensilla, interior of reticulations with fine, granulate surface (visible only in slide-mounts under high magnification); mid-lobe of mesoscutum with 2 pairs of long setae and about 40 short setae, side lobes each with 2 long and 1 short setae; scutellum with 2 pairs of long setae and two sensilla directly posterior to the anterior pair of setae, scutellar sensilla slightly posterior to middle of scutellum; mesotibial spur 0.8× mesobasitarsus; metatibial spur 0.6× metabasitarsus.
Fore wing. (Fig. 10) 2.3× as long as broad; costal cell with 1 complete row of dorsal setae and 2 rows of ventral setae, the posterior row extending from under the proximal end of the marginal vein almost to stigma, costal cell 1.2× longer than marginal vein; submarginal vein with 5–6 setae; marginal vein with 10 setae along the margin; stigmal vein short with stigma rounded; delta region proximal to linea calva with one complete line of 13–15 setae and 2–6 additional setae in angle with marginal vein, linea calva with no dorsal setae at its posterior edge; wing distal to linea calva with dense, evenly spaced, dorsal setae and much smaller ventral setae.
Hind wing. (Fig. 11)4.3× as long as broad, marginal fringe 0.26× wing width.
Metasoma. (Figs 4, 6, 12) 1.2× as long as mesosoma; ovipositor inserted at middle of metasoma, slightly exerted distally, 1.3× as long as meso- and metatibiae; third valvula one-third length of ovipositor.
Color. (Figs 2, 4, 6) Head and mesosoma dark brown to black; legs with procoxa yellowish white, meso- and metacoxae dark brown to black, femora yellowish white, protibia yellowish white, mesotibia yellowish white with center greyish, metatibia dark grey to black base pale, tarsi pale brown with tips and metabasitarsus greyish brown; metasoma with base (T1 and T2), apex, and venter yellow, remainder brown; antennae yellowish white; compound eyes burgundy, and ocelli red in life, both silver-colored in dried specimens.
Male (Figs 1, 3, 5, 7, 9, 14). Similar to female except:
Head. (Figs 1, 7) Antenna with scape swollen in middle, 3.4 as long as broad, maximum width 3× distal end width, with 3–5 volcano-shaped secretory pores in line on ventral surface, pedicel 2.1× as long as broad, F1 anneliform, 1.8× as wide as long, F2 1.5× as broad as long, F3 2.0× as long as broad, club 3.9× as long as broad, 2.1× as long as F3, and with 4–6 longitudinal sensilla.
Metasoma. (Figs 3, 5, 14) About the same length as mesosoma, phallobase of genitalia including digiti 4.5× longer than broad, digiti about twice longer than broad and with two apical denticles.
Color. (Figs 1, 3, 5) Legs with procoxa grey, pro- and mesofemora sometimes with darkened centers; metasoma brown with base and apex yellow; scape dark greyish brown with greyish yellow distal tip, pedicel greyish yellow.
(card-mounted, deposited in USNM, USNM ENT 00703637).“China: Liaoning, Xiuyan | 40°18'N, 123°14'E | 11.vii.2007, K. Hoelmer || ex: Aphis glycines | on: soybean | plots 1/3, 2007/007 || From Lab Culture | USDA-ARS-BIIRU | Newark, Delaware”
(USNM, TAMU, BMNH). 30 card-mounted and 4 slide-mounted ♀♀, 14 card-mounted and 4 slide-mounted ♂♂ with same data as holotype. 13 card-mounted and 4 slide-mounted ♀♀ and 9 card-mounted and 3 slide-mounted females: China, Liaoning, Xiuyan, 40°20'N, 116°6'E, 12.vii.2007, K. Hoelmer, ex: Aphis glycines on: soybean, plot 2, 2008/008, from Lab Culture, USDA-ARS-BIIRU, Newark, Delaware, all bearing TAMU accession numbers.
In the field, Aphis glycines is the only known host. In laboratory experiments, Aphelinus glycinis parasitizes Aphelinus glycines and closely related species in the genus Aphis.
This species is named for the host from which it was collected. The species epithet is a noun in genitive case.
Aphelinus glycinis is closest to Aphelinus engaeus and Aphelinus ficusae Prinsloo and Neser based on our matrix of traits (Table 2). Aphelinus glycinis differs from Aphelinus engaeus in having elongated third funicle segments in males and females, and it differs from Aphelinus ficusae in having an ovipositor more than 1.2× as long as the mesotibia and grey procoxa in males. It also differs from these species in its aphid hosts and geographical distribution. Aphelinus glycinis is a specialist on Aphis species close to Aphis glycines, but Aphelinus engaeus is reported from Schizaphis graminum (Rondani) and Sitobion ochnearum (Eastop) and Aphelinus ficusae was reared from an undetermined aphid on Ficus sycomorus (
Aphelinus glycinis sp. n. paratype specimens in 95% ethanol. 1 male antennae and face 2 female antennae and face 3 male, lateral view 4 female, lateral view 5 male, ventral view 6 female, ventral view.
Aphelinus glycinis sp. n., slide-mounted paratypes. 7 male antenna (TAMU x0616203) 8 female antenna (TAMU x0616201) 9 male fore wing (TAMU x0616206) 10 female fore wing (TAMU x0616201) 11 female hind wing (TAMU x0616204) 12 female metasoma (TAMU x0616204) 13 female mesosoma (TAMU x0616211) 14 male genitalia (TAMU x0616206).
urn:lsid:zoobank.org:act:1132B1E4-8F2E-4FF3-9E6B-30FAFD497EA1
http://species-id.net/wiki/Aphelinus_rhamni
Figs 15– 28Females. Head and mesosoma dark brown to black; legs with coxae dark brown to black, profemur dark grey with pale apex, mesofemur dark grey to black, metafemur white, protibia white with pale greyish base, mesotibia dark grey to black with pale base and apex, and metatibia dark grey to black with pale base; metasoma yellowish brown with base and apex yellow; antennae yellow with basal half of scape and pedicel sometimes greyish; F3 quadrate; club 2.8 times as long as broad. Males similar except scape swollen in middle, 3× broader in middle than at distal end, with 2 or 3 volcano-shaped secretory pores; scape dark yellowish grey, pedicel pale greyish yellow; club 3.3 times as long as broad.
Female (Figs 16, 18, 20, 22, 24, 25, 26, 27).
Body length. 0.75–0.94 (Holotype 0.87 mm).
Head. (Figs 16, 22) Head 1.2× as broad as high in frontal view, about as broad as mesosoma; frontovertex 0.4× head width and as broad as scape length; posterior ocelli 0.5× their diameter from eye margin, 3.0× their diameter from one another, and 0.33× their diameter from occipital margin; mandible with 2 acute teeth and a broad truncate surface below the teeth; antennae as in Fig. 21 with scape 4.8 longer than broad, pedicel 1.8× as long as broad, F1 anneliform, F2 1.5× as broad as long, F3 quadrate, club 2.8× as long as broad and 3.3× times longer than F3, with 4–6 longitudinal sensilla.
Mesosoma. (Figs 18, 20, 27) Mesoscutum and scutellum with fine reticulate sculpture, longest diameter of reticulations approximately twice the diameter of scutellar sensilla, interior of reticulations with fine, granulate surface (visible only in slide-mounts under high magnification); mid-lobe of mesoscutum with 2 pairs of long setae and 35–40 short setae, side lobes each with 2 long and 1 short seta; scutellum with 2 pairs of long setae, pair of scutellar sensilla directly posterior to the anterior pair of setae and slightly posterior to middle of scutellum; mesotibial spur equal in length to mesobasitarsus; metatibial spur 0.5× metabasitarsus.
Fore wing. (Fig. 24) 2.2× as long as broad ; costal cell with 1 row of dorsal setae and two rows of ventral setae, the posterior row extending from under the proximal end of the marginal vein almost to stigma, costal cell 1.1× as long as marginal vein; submarginal vein with 5 setae, marginal vein with 10 setae along the anterior margin; stigmal vein short with stigma rounded; delta region proximal to linea calva with one complete line of 13–15 setae and 2–6 additional setae in angle with marginal vein, linea calva with no setae at its posterior edge; wing distal to linea calva with evenly spaced, dense dorsal setae and much smaller ventral setae.
Hind wing. (Fig. 25)3.9× longer than broad, marginal fringe 0.23× wing width.
Metasoma. (Figs 18, 20, 26) 1.4× as long as mesosoma; ovipositor inserted at middle of metasoma, slightly exerted distally, 1.3× longer than metatibia and mesotibia; third valvulae one-third the length of ovipositor.
Color. (Figs 16, 18, 20) Head and mesosoma dark brown to black; legs with coxae dark brown to black, profemur dark grey with pale apex, mesofemur dark grey to black, metafemur white, protibia white with pale greyish base, mesotibia dark grey to black with pale base and apex, and metatibia dark grey to black with pale base; metasoma yellowish brown with venter of T1 and apex yellow; antennae yellow sometimes with basal half of scape and pedicel greyish yellow; compound eyes dark burgundy and ocelli red in life, both silver-colored in dried specimens.
Male (Figs 15, 17, 19, 21, 23, 28). Similar to female except:
Body length. 0.66–0.78 mm.
Head. (Figs 15, 21) Antenna with scape swollen in center, 3.1× as long as broad, maximum width 3× width at distal end, with 2–3 volcano-shaped secretory pores in single line on ventral surface, pedicel 1.8× as long as broad, F1 subquadrate, 1.1× as broad as long, F2 shorter, 1.4× as broad as long, F3 trapezoidal, 1.1× longer than width at apex, 1.5× as long as wide at base, club 3.3× as long as broad, with 4 longitudinal sensilla.
Metasoma. 0.7× length of mesoma
Color. (Figs 15, 17, 19) Scape dark yellowish grey, pedicel pale greyish yellow, base of metasoma pale brown and with yellow region at apex smaller.
(card-mounted, deposited in USNM, USNM ENT 00763638). “China, Daxing (Beijing) | 39°48'N, 116°28'E | 10.ix.2005, K. Hoelmer || ex: Aphis glycines | on: Rhamnus sp. | 2005/005 || From Lab Culture | USDA-ARS-BIIRU | Newark, Delaware”
(USNM, TAMU, BMNH). 33 card-mounted and 6 slide-mounted females, 19 card-mounted and 3 slide-mounted males with same data as holotype. 9 card-mounted and 2 slide-mounted females, 6 card-mounted and 2 slide-mounted males: China: Daxing (Beijing), 39°48'N, 116°28'E, 10.iv.2004, W. Meikle coll., ex: Aphis glycines on Rhamnus sp., 2004/008, from lab culture, USDA-ARS-BIIRU, Newark, Delaware.
In the field, Aphis glycines is the only known host. In laboratory experiments, Aphelinus rhamni parasitizes Aphelinus glycines and closely related species in the genus Aphis, and rarely Rhopalosiphum padi L. and Schizaphis graminum.
This species is named for the primary host plant of the aphid species from which it was collected. The species epithet is a noun in genitive case.
Aphelinus campestris and Aphelinus gossypii are the closest described species to Aphelinus rhamni based on our matrix of traits (Table 2). Aphelinus rhamni differs from both species in having a more elongate club and in coloration of the metatibia. Aphelinus rhamni has a much narrower host range than Aphelinus gossypii, which is reported from at least 18 species of aphids in 10 genera and two tribes, including species which Aphelinus rhamni does not parasitize in laboratory experiments.
Aphelinus rhamni sp. n., paratype specimens in 95% ethanol. 15 male antennae and face 16 female antennae and face 17 male, lateral view 18 female, lateral view 19 male, ventral view 20 female, ventral view.
Aphelinus rhamni sp. n., slide-mounted paratypes. 21 male antenna (TAMU x0616221) 22 female antenna (TAMU x0616215) 23 male fore wing (TAMU x0616217) 24 female fore wing (TAMU x0616215) 25 female hind wing (TAMU x0616129) 26 female metasoma (TAMU x0616214) 27 female mesosoma (TAMU x0616129) 28 male genitalia (TAMU x0616217).
urn:lsid:zoobank.org:act:F4B3A880-2136-474C-815C-13406F2A48A0
http://species-id.net/wiki/Aphelinus_coreae
Figs 29– 42Females. Head and thorax dark brown to black; legs with coxae dark brown to black, profemur dark grey with distal half pale, mesofemur dark grey to black, metafemur pale yellowish white, protibia pale yellowish white to somewhat fuscous, mesotibia dark grey to black with distal half pale, and metatibia dark grey to black with pale base; metasoma dark brown with base and apex yellow; antennae yellow; F3 quadrate. Males similar except scape swollen in middle, 2.0× as broad in middle than at distal end, with two or occasionally three circular secretory pores in the middle of a shallow depression on ventral surface, scape dark yellowish grey with distal half yellow, pedicel greyish yellow.
Female (Figs 30, 32, 34, 36, 38, 39, 40, 41).
Body length. 0.80–0.93 (Holotype 0.93 mm).
Head. (Figs 30, 36) Head 1.3× as broad as high in frontal view, about as broad as mesosoma; frontovertex 0.4× head width and as broad as scape length; posterior ocelli approximately their own diameter from eye margin, 5× their diameter from one another, and 0.5× their diameter from occipital margin; mandible with two acute teeth and a broad truncate surface below the teeth, ventral tooth sometimes not distinct; antennae as in Figs 30 and 36 with scape 4.0× as long as broad, pedicel 1.6× as long as broad, F1 anneliform, F2 1.4× as broad as long, F3 subquadrate or very slightly broader than long, club 3.75× as long as broad and 3.5× longer than F3, with 7–8 linear sensilla.
Mesosoma. (Figs 32, 34, 41) Mesosoma and scutellum with fine reticulate sculpture, longest diameter of reticulations approximately 2–3× diameter of scutellar sensilla; interior of reticulations with fine, granulate surface (visible only in slide-mounts under high magnification), mid-lobe of mesoscutum with 2 pairs of long setae and about 40–60 short setae, side lobes each with 2 long and 1–2 short setae; scutellum with 2 pairs of long setae; pair of scutellar sensilla approximately equidistant from anterior and posterior pairs of long setae; mesotibial spur 1.1× mesobasitarsus; metatibial spur 0.6× metabasitarsus.
Fore wing. (Fig. 38) 2.2× as long as broad; costal cell with 1 row of dorsal setae and 2 rows of ventral setae, the posterior row extending from under the distal end of the submarginal vein almost to stigma, costal cell 1.3× as long as marginal vein; submarginal vein with 5 setae; marginal vein with 12 setae along the anterior margin; stigmal vein short with stigma rounded; delta region proximal to linea calva with one complete line of 12–13 setae and 2–5 additional setae in angle with marginal vein, linea calva with no setae at its posterior edge; wing distal to linea calva with evenly spaced, dense dorsal setae and much smaller ventral setae.
Hind wing. (Fig. 39) 3.9× as long as broad, marginal fringe 0.26× wing width.
Metasoma. (Figs 32, 34, 40) 1.1× as long as mesosoma; ovipositor inserted at basal third of metasoma, slightly exerted distally, 1.4× as long as metatibia or mesotibia; third valvula 0.28× length of ovipositor.
Color. (Figs 30, 32, 34) Head and mesosoma dark brown to black; legs with coxae dark brown to black, profemur dark grey with distal half pale, mesofemur dark grey to black, metafemur pale yellowish white, protibia pale yellowish white to somewhat fuscous, mesotibia dark grey to black with distal half pale, and metatibia dark grey to black with pale base; metasoma dark brown with base and apex yellow; antennae yellow; compound eyes dark burgundy, ocelli red in life, both silver-colored in dried specimens.
(Figs 29, 31, 33, 35, 37, 42). Similar to female except:
Body length. 0.68–0.81 mm.
Head. (Figs 29, 35) Antenna with scape swollen in center, 3.3× as long as broad, maximum width 2× distal width, with 2 or 3 circular, secretory pores in the middle of a shallow depression on ventral surface, pedicel 2.0× longer than broad, F1 and F2 1.4× broader than long, F3 rectangular, 1.3× as long as wide at apex, club 3.2× as long as broad, with 3–4 longitudinal sensilla.
Metasoma. (Figs 31, 33, 42) 1.5× length of mesoma.
Color. (Figs 29, 31, 33) Scape dark yellowish grey with distal half yellow, pedicel greyish yellow.
Holotype female (card-mounted, deposited in USNM, USNM ENTO 00763639). “Korea, Gyeongsangnam Province, Miryang |35°30'N, 128°44'E | 11.viii.2009, K. Hoelmer || ex: Aphis glycines | on: soybean | 2009/011 || From Lab Culture | USDA-ARS-BIIRU | Newark, Delaware”
(USNM, TAMU, BMNH). 13 card-mounted and 3 slide-mounted ♀♀ and 9 card-mounted and 5 slide-mounted ♂♂ with same data as holotype.
None.
In the field, Aphis glycines is the only known host. In laboratory experiments, Aphelinus coreae parasitizes Aphelinus glycines and other species in the genus Aphis, and occasionally Rhopalosiphum padi and Schizaphis graminum.
This species is named for its country of origin. The species epithet is a noun in genitive case.
Aphelinus campestris and Aphelinus gossypii are the closest described species Aphelinus coreae based on our matrix of traits (Table 2). Aphelinus coreae differs from both species in having a more elongate club in females and in coloration of the scape and mesotibia. Like Aphelinus rhamni, Aphelinus coreae has a much narrower host range than Aphelinus gossypii. Aphelinus coreae is very close to Aphelinus rhamni, but male Aphelinus coreae have shorter clubs and, as noted in the key, the two species differ in coloration of scape and mesotibia. Although difficult to distinguish, these species are reproductively isolated in laboratory crosses. Their DNA differs by 2130 fixed substitutions and 293 indels across 1.8 megabases of homologous DNA sequence. They also differ in host specificity: Aphelinus coreae parasitizes species of Aphis, e.g. Aphelinus nerii Boyer de Fonscolombe and Aphelinus rumicis L., not parasitized by Aphelinus rhamni in laboratory experiments.
Aphelinus coreae sp. n., paratype specimens in 95% ethanol. 29 male antennae and face 30 female antennae and face 31 male, lateral view 32 female, lateral view 33 male, ventral view 34 female, ventral view.
Aphelinus coreae sp. n., slide-mounted paratypes. 35 male antenna (TAMU x0616221) 36 female antenna (TAMU x0616215) 37 male fore wing (TAMU x0616217) 38 female fore wing (TAMU x0616215) 39 female hind wing (TAMU x0616129) 40 female metasoma (TAMU x0616214) 41 female mesosoma (TAMU x0616129) 42 male genitalia (TAMU x0616217).
Alyssa Mann, undergraduate student at Texas A&M University, helped with digital imaging and preparation of figures. Kathryn Lanier, USDA-ARS, Newark, Delaware, reared the cultures of Aphelinus coreae, Aphelinus glycinis and Aphelinus rhamni.We thank István Miko for his generous help in reading the ms and helping us to standardize our terminology according to the Hymenoptera Anatomy Ontology project, and for providing the table of uri’s for anatomical terms used in the paper. We thank the editors and two anonymous reviewers for their suggestions. This research was supported in part by funding from the North Central Soybean Research Program (KRH) and NSF award DEB 0730616 (JBW).