(C) 2012 Milagros Dalmazzo. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The nesting biology of Augochlora (Augochlora) amphitrite (Schrottky) in a natural reserve in the Province of Buenos Aires, Argentina, is described. The species nests in decaying wood. Two types of nest architecture were found, which differed according to the substrate where they were built, either soft or hard wood. Nests in soft wood had the cells grouped in clusters surrounded by a cavity, and the clusters were supported by a varying number of pillars. Nests constructed in decomposing portions of cracks in otherwise hard wood had the cells constructed against the walls, without any pillars or surrounding cavity. Cells of both types of nests were oriented in all directions, without any detectable pattern. Measurements and characteristics of the nests are tabulated and compared to those known for other species of Augochlora s. str. Behavioral observations of active nests are indicative of a social division of tasks in Augochlora amphitrite. Such observations include nests with several females, some of which were never observed outside the nests, females with different degrees of wear and of ovary development, and at least one female that actively collected pollen which had much worn mandibles and wings, and undeveloped ovaries, all characteristics of the worker caste in social halictids.
Augochlorini, Augochlora, nesting biology, social behavior, Pampean Region, Argentina
The bee tribe Augochlorini has an exclusively New World distribution, with maximal diversity in the Neotropics. This tribe is of particular interest because of the diversity of many of its biological traits, at the genus and species level, as well as within species. The social behavior in this group varies from solitary to primitively eusocial, with various degrees of sociality and transitions, including the origin of solitary behavior from eusocial ancestors (
Augochlora is one of the more diverse genera within the tribe, with nearly 120 named species, classified in two subgenera, Oxystoglossella and Augochlora s. str. (
The two subgenera of Augochlora are considered as behaviorally divergent (
This contribution describes the structure of nests of Augochlora amphitrite, and presents information on the nesting biology of the species. The data are compared to those known for other species of the subgenus.
Methods Study siteThe nests were studied in the reserve Refugio Natural Educativo “Ribera Norte" (34°28'10"S, 58°29'40"W), San Isidro, province of Buenos Aires, Argentina. This reserve is on the west margin of the Río de La Plata, and preserves a relict of gallery forest with typical riverine vegetation, including trees such as Ocotea acutifolia (Nees) Mez (Lauraceae), Nectandra falcifolia (Nees) J.A. Castigl. Ex Mart. Crov. & Paccinini(Lauraceae), Pouteria salicifolia (Spreng.) Radlk.(Sapotaceae), Allophylus edulis (A. St.-Hill., A. Juss. & Cambess.) Hieron. ex Niederl. (Sapindaceae), Sebastiania brasiliensis Spreng. (Euphorbiaceae), Sapium haematospermun Müll. Arg. (Euphorbiaceae) and Blepharocalyx tweediei (Hook, et Arn.) O Berg. (Myrtaceae) (
A nesting site of Augochlora amphitrite was discovered in March 2008, near the end of summer. Nests were observed during seven days (35 work hours), from March 12 to April 30, when the nests were excavated. Another nesting site was located the following year in February. It was observed during three days (15 work hours), from February 7 to 14, when the nests were excavated. Although nests were not found in spring, adults flying over flowers (September to November) were collected and kept for dissection.
The activity of the bees was recorded following the methods described by
The methodology described by
The day of nest excavation, arriving bees as well as those found within the nest, were fixed in Kahle’s solution. Presence of pollen loads, ovarian development, and presence of fat tissue, were recorded. Length of the body, maximum width of the eye and maximum width of the gena were taken. All measurement are in millimeters.
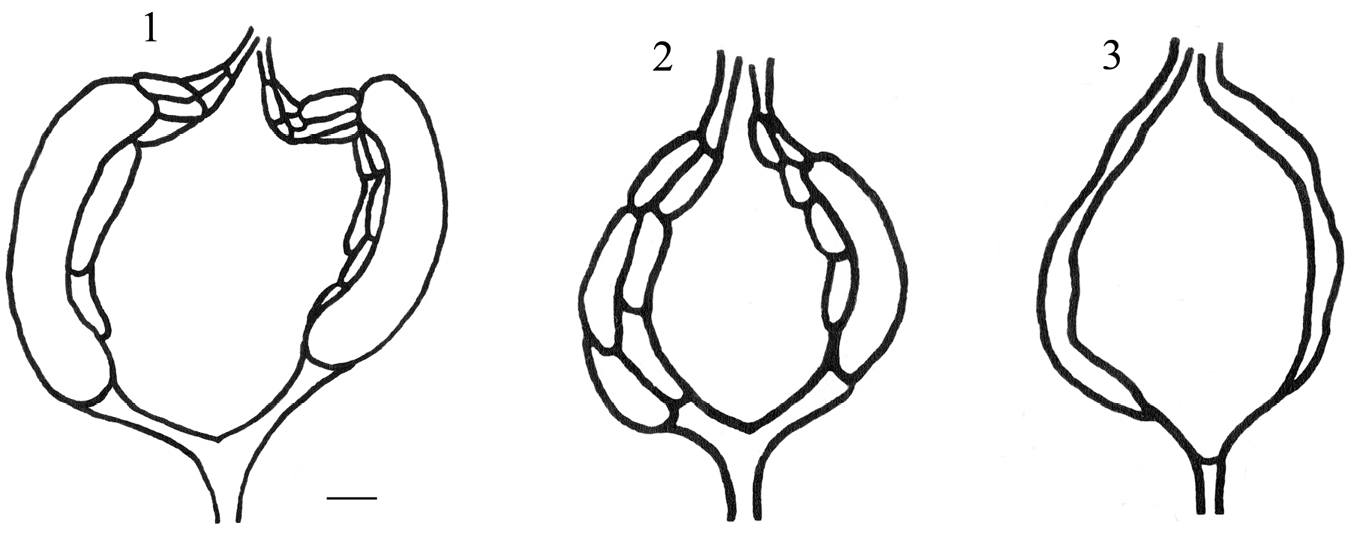
Three groups of females are recognized according to their ovarian development. The classification of
The degree of wear of mandibles and wings is indicated in a scale from 0 (intact mandibles and wings) to 3 (much worn mandibles and tattered wings).
Classification of ovaries according to their development. 1 group A, developed ovaries with mature eggs ready to be deposited 2 group B, developed ovaries without mature eggs. 3 group C, ovaries not developed. Scale line: 0.1 mm.
An aggregation of 18 nests was found in a fallen trunk of Salix sp. (Salicaceae) on March 12, 2008. The trunk, 3 m long and 0.8 m in diameter, was in an advanced state of decomposition, with soft wood colonized by fungi and various arthropods. Half of the trunk surface was covered by the plant Commelina diffusa Burm. f. (Commelinaceae), but the nests were on the uncovered surface, occupying an area of 0.60 m2 on the upper and lateral parts. The nest entrances received sunlight from 11:30 to 15:00, being shaded by surrounding trees the rest of the day.
Three nests were found in railroad sleepers made from Schinopsis sp. (“quebracho colorado”) (Anacardiaceae) on February 7, 2009. The sleepers (1.0 m long, 0.4 m wide, and 0.15 m thick) lay on the ground, forming the visitors trail in the wettest parts of the reserve. Schinopsis wood is well known for its hardness. The nest entrances were located in knots and cracks, where decomposition had begun to soften the wood. The entrances were on the upper and lateral surfaces, occupying an extension of 0.50 m2, and receiving sunlight from 11:00 to 15:00 hours.
Nest architectureNests on Salix and on Schinopsis differ considerably in their architecture, mainly in the distribution and arrangement of the cells.
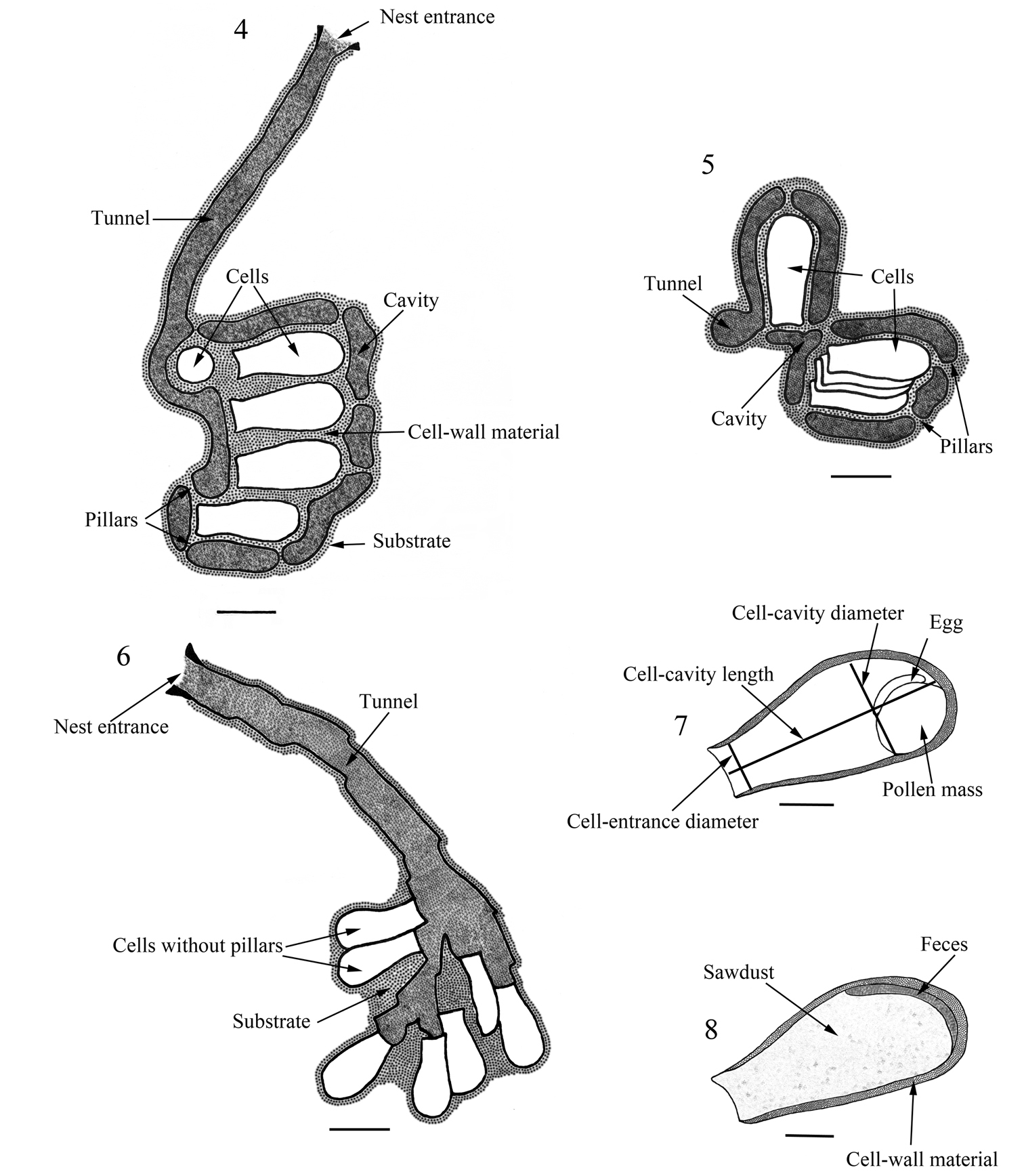
Nest entrances on the trunk of Salix, separated by a minimum distance of 10 cm, presented a ring of compacted sawdust 0.75–1.00 cm in diameter (x‒= 0.85 ± 0.08, n= 8) of the same color of the trunk surface. Active nests sometimes presented loose particles beyond the ring, which came from broken nest plugs. The tunnels, all unbranched, penetrated toward the interior of the trunk. They had a length of 7.00–15.00 cm (x‒= 9.67 ± 2.56, n= 8), and a diameter of 0.45–0.50 cm (x‒= 0.46 ± 0.03, n= 8); their smoothed walls were lined with substrate particles. Each tunnel led to a cluster of 2–10 cells (x‒= 5 ± 2, n= 18), irregularly oriented, supported within a cavity by pillars. Two kinds of pillars were observed, those that were remaining parts of the substrate not excavated, and others, more frequent, made of compacted sawdust. The clusters were retrieved intact (Figs 4–5).
Nests on Schinopsis had shorter tunnels, 2.00–5.00 cm long (x‒= 3.10 ± 1.36, n= 3). The soft material of the cracks was used for cell construction. The cells were in small groups or isolated, but without any pillars, and lying against the hard wood, with no surrounding cavity, taking advantage of masses of soft substrate within the crack (Fig. 6). Nests had 8–19 cells (x‒= 13 ± 5, n= 3).
Cells of all nests were constructed with compacted particles of ground wood. The external surface was irregular, and the internal surface smooth and shiny, lined with a waxy substance. The cells were ovoid, with the lower surface slightly flattened (Fig. 7); the inner cavity was 0.80–1.45 cm long (x‒= 1.07 ± 0.13, n= 72), 0.30–0.60 cm in diameter (x‒ = 0.46 ± 0.06, n= 72), and 0.25–0.45 cm in cell entrance diameter (x‒= 0.36 ± 0.04, n= 72); the cell wall was 0.05–0.30 cm in thickness (x‒= 0.09 ± 0.04, n= 72). The cell plugs were made with the same material as the cell walls, 0.15 cm in thickness, dish-shaped, with the outer surface concave. Table 1 summarizes the architectural characteristics of Augochlora amphitrite, comparing them to other species of Augochlora s. str. known to date.
Architechtural characteristics of nests of Augochlora s. str. Measurements are given in cm (mean ± SD). Data for species other than Augochlora amphitrite, from
| Augochlora amphitrite | Augochlora isthmii | Augochlora alexanderi | Augochlora esox | Augochlora pura | Augochlora hallinani | Augochlora sidaefoliae | Augochlora smaragdina | |
|---|---|---|---|---|---|---|---|---|
| Nest entrance (diameter) | 0.85 ± 0.08 | 0.34 ± 0.04 | 0.36 | 0.50 | ||||
| Tunnel | ||||||||
| Diameter | 0.46 ± 0.03 | 0.64 ± 0.11 | 0.52 | 0.80 | ||||
| Length | 9.67 ± 2.56 | 8.60 ± 0.65 | 5.60 | 20 | 2.50 | 5.00 | ||
| Cells | ||||||||
| Arrangement | Clusters supported by pillars. Cells isolated or in groups against substrate without pillars or surrounding cavity | Isolated along the tunnel | Isolated along the tunnel | In groups against substrate without pillars or surrounding cavity | Clusters supported by pillars. Planiform. Along a tunnel. Intermediate forms. | Isolated along the tunnel | Cluster supported by pillars. | In a column along the tunnel |
| Orientation | Radiated in all directions | Radiated in all directions | Radiated in all directions | Radiated in all directions. Horizontal. | Parallel, sub-horizontal. Radiated in all directions | Horizontal | Horizontal | |
| Inner length | 1.07 ± 0.13 | 1.39 ± 0.11 | 0.88 | 1.2 | 0.84–1 | 1.50(outer dimensions) | ||
| Inner max. diameter | 0.46 ± 0.06 | 0.63 ± 0.06 | 0.43 | 0.5 | 0.4–0.6 | |||
| Neck diameter | 0.36 ± 0.04 | 0.48 ± 0.03 | 0.35– 0.45 | |||||
| Wall thickness | 0.09 ± 0.04 | 0.1–0.4 | 0.2 |
Nests of Augochlora amphitrite. 4–5 nest on Salix sp. 4 section of nest in lateral view 5 section of same nest in upper view 6 nest on Schinopsis sp., section of nest in lateral view 7 cell with pollen mass and egg, indicating taken measurements 8 cell filled with feces and sawdust. Scale lines: Figs 4–6: 10 mm, Figs 7–8: 5 mm.
Nests collected from Salix in April had their cells filled with compacted sawdust; they had feces deposited on the posterior portion, oriented toward the bottom of the cell (Fig. 8).
Nests collected from Schinopsis in February were active, and the cell contents consisted of pollen masses with eggs, larvae (in various stages of development), pupae, and a few cells with feces and filled with sawdust (Table 2). The pupae were all males. The pollen mass, placed near the bottom on the flattened surface of the cell, was slightly wider than long (0.40 × 0.35 cm), 0.43 cm high, and rather spherical, except for the flattened resting surface. The whitish egg was deposited on top of the mass, oriented along the longitudinal axis of the cell (Fig. 7).
Cell contents of nests of Augochlora amphitrite. Po (pollen), E (egg), Lpo (larva with pollen), pdL (pre-defecating larva), Pu (pupa), F, s (Feces, filled with sawdust), f (female), m (male).
| Cell contents | Number of cells | Adults | ||||||
|---|---|---|---|---|---|---|---|---|
| Po | E | Lpo | pdL | Pu | F, s | |||
| Active nests | ||||||||
| Nest 1 | 7 | 3 | 1 | 4 m | 15 | 5f - 2m | ||
| Nest 2 | 7 | 1 | 3 | 2 | 3 m | 3 | 19 | 2f - 1m |
| Nest 3 | 4 | 3 | 1 | 8 | 1 f | |||
| Inactive nests | 72 | 72 | 6 f | |||||
Females observed leaving and entering nests in March-April did not carry pollen loads. Activity began soon after the sunlight hit the trunk; before that, the entrances were covered with closed tumuli. Flights were inconstant, and up to three females were seen leaving and entering the same nest. The females spent 10–15 minutes perching on the surrounding vegetation, where flying males were also observed. Returning females had erratic flights, and inspected cracks and small holes in the trunk.
The three nests collected in February were active. Foraging activity began 15–20 minutes after the sunlight hit the entrances (around 11:20). A female pushed the plug of sawdust with its hind legs, scattering the particles 2–3 cm around the tumulus. After that the female remained at the entrance, with only its head visible, for 3–5 minutes before departing. After 7–10 minutes the same female came back to the nest laden with pollen. Usually, as soon as a female left the nest, another one showed its head at the entrance. When disturbed, the female turned around, plugging the hole with its metasomal terga. Activity continued for approximately 4 hours until no more sunlight bathed the nests (around 15:00). Nest 1 had five females, four of which were captured when returning to the nest (two with, and two without pollen loads); the fifth female was never observed outside the nest and was captured when it was extracted. Another nest had two females; only one of them was observed collecting pollen. The third nest had a single female. Recently emerged males were found in two nests, and males were seen flying in the surroundings of the nesting area and on flowers of Ludwigia (Onagraceae), 50 cm away from the nests.
DissectionsInactive nests (end of summer). The six fixed females had slender, undeveloped ovaries (group C, Fig. 3) and mandibles and wings without signs of wear (class 0). All specimens had abundant fat tissue, in the form of small, whitish spheres. None carried pollen loads. Measurements (length of body – maximum width of eye – maximum width of gena): 9.0–0.5–0.4; 10.0–0.5–0.4; 10.0–0.5–0.4; 11.0 –0.6–0, 6; 8.0–0.6–0.5; 11.0–0.8–0.8.
Active nests (summer)Nest 1 (5 females). Three females were group A, had unworn mandibles (class 0), and slightly worn wings (class 1); two of them were seen carrying pollen loads to the nest; measurements, 9.0–0.6–0.5; 10.0–0.6–0.8; 9.0–0.6–0.7. One female was group B, with unworn mandibles (class 0), and slightly worn wings (class 1); this female was never observed outside the nest; measurements, 12.0–0.7–0.9. One female was group C, had worn mandibles and wings (class 2); this female carried pollen loads; measurements, 9.5–0.6–0.5. Nest 2 (2 females). Both were group A, with slightly worn mandibles (class 1) and worn wings (class 2); one of them carried pollen loads to the nest. Measurements: 9.5–0.5–0.4; 11.0–0.6–0.7.
Nest 3 (1 female). It was group A, with slightly worn mandibles (class 1) and worn wings (class 2). This female was not observed outside the nest and was captured during excavation. Measurements: 11.0–0.6–0.7.
Spring flying adults. Fourteen females were captured in spring (1, September; 7, October; 6, November). All had developed ovaries (2, group A; 12, group B), and unworn wings and mandibles (class 0). None contained fat tissue. Maximum and minimum values for these females were: length of body, 8.0–12.5, width of eye, 0.5–0.7, and width of gena, 0.5–0.9.
Discussion and conclusionsThe nests of Augochlora amphitrite presented two types of nest architecture according to the substrate where they were built. Common features to both types were the entrance surrounded by a ring of compacted sawdust, and the unbranched tunnels leading to the cells. Cells of all nests had the same structure, and similar proportions to those of other species of Augochlora s. str. (Table 1).
Nests constructed in the thick trunk of Salix, with a large mass soft wood, had the cells grouped in clusters surrounded by a gallery of similar diameter to that of the tunnel, and supported by a varying number of pillars. Nests constructed in the decomposing parts of the cracks and knots of the hard wood of Schinopsis had the cells toward the end of the tunnel, constructed against the hard walls, without any pillars or surrounding cavity. In both cases the number of cells was variable and the orientation of the cells irregularly radiated.
Cluster nests are known for Augochlora pura and Augochlora sidaefoliae, while studied nests of Augochlora isthmii, Augochlora alexanderi, Augochlora hallinani and Augochlora smaragdina had tunnel nests with sessile cells distributed along the tunnel, and Augochlora essox had nests with grouped cells, but not forming clusters (Table 1) (
The daily activity pattern of the females was limited by the forest environment where the bees were studied. Females left the nests to collect pollen during a period of 3.5–4 hours, while the sunlight hit the nesting site.
The annual cycle in the study area was typical of the cycle of most halictids in temperate regions, although the winters in the study area are mild and the temperatures in July are rarely freezing. Activity begins in spring (September-October), when posthibernating females begin to visit flowers. Females captured at this time showed well developed ovaries and would be the foundresses of the first nests. The activity continues until mid March, when the nests become inactive and females of the last generation are looking for hibernacula. Females captured at this time had undeveloped ovaries and abundant fat tissue.
Nests studied in summer (February) contained larvae in various stages of development, male adults, male pupae, and adult females, which correspond at least to the first brood of the foundress (Table 2). Although possible, we are not certain whether more generations are bred between spring and mid-summer. A further brood is produced by the end of summer, so at least two broods are produced during the activity cycle. Although most females collected in February had developed ovaries, one female, from nest 1, had the ovaries undeveloped and actively collected pollen. It also had worn mandibles and wings, all characteristics of the worker caste in social halictids (
Values taken from the fixed females show size variation among females with enlarged ovaries. The female that was never observed outside the nest in multi-female nest 1 was distinctly larger than the others in the same nest. It also had an allometrically enlarged head, with a broad gena. Females of Augochlora amphitrite have distinct cephalic polymorphism (
Although the number of studied nests is very low, the information recovered is suggestive of social behavior in Augochlora amphitrite.
We thank the personnel of the reservation Refugio Natural Educativo Ribera Norte, Guillermo Bryant, Ricardo Camiña, and Gustavo Suárez, for their invaluable help and cooperation, and William T. Wcislo and Laurence Packer for helpful comments on the manuscript. This study was supported by grants ANPCyT, Argentina, 2007-1238 préstamo BID, and CONICET, Argentina, PIP 2011-0288.