(C) 2012 Aline Candida Ribeiro Andrade e Silva. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The nesting biology and social behavior of the euglossine bee species Euglossa melanotricha was analyzed based on the monitoring of eight nests found in man-made cavities and transferred to observation boxes. Euglossa melanotricha females usually construct their nests in cavities in the ground, in buildings, or in mounds. In this study, we present new data on the nesting biology of Euglossa melanotricha. The process of reactivation of nests was commonly observed with one to three females participating in the reactivation. The duration of the process of reactivation ranged from 10 to 78 days (n = 31) and were longer during the rainy season. Time spent (in days) for provisioning, oviposition and closing a single cell was higher in reactivations that occurred during the dry period.151 emergences were observed (39 males and 112 females). 90 (80.3%) of the emerged females returned to the natal nest, but only 35 (38.9%) remained and actively participated in the construction and provisioning of cells.The other 55 abandoned the nests after several days without performing any work in the nest. Matrifilial nest structure was regulated by dominance-subordinate aggressive behavior among females, where the dominant female laid almost all eggs. Task allocation was recognized by behavioral characteristics, namely, agonism and oophagy in cells oviposited by other females. Euglossa melanotricha is multivoltine and its nesting is asynchronous with respect to season. Our observations suggest a primitively eusocial organization. These observations of Euglossa melanotricha provide valuable information for comparison with other species of Euglossa in an evolutionary context.
Orchid bees, nest structure, nesting behavior
The bees of the tribe Euglossini are the only members of the corbiculate bees that do not form large colonies with a typical queen and worker caste (
A diversity of nesting behavior is observed in the different species of Euglossa. Some species construct aerial nests (
Observations of the intranidal behavior of Euglossa carolina females (cited as Euglossa cordata) during nest reactivation have clearly demonstrated that the mother or a sister becomes the reproductive female and the other females perform other nest related tasks (
Similar social organization have been reported by
Euglossa melanotricha Moure, 1967 is a medium-sized bee (body length 13 mm) commonly found in open areas of savanna habitat in Brazil and Bolivia. The species is abundant in the Serra do Espinhaço mountain range in the Brazilian states of Minas Gerais and Bahia, but is rare or absent in areas of low altitude or dense forest (
This long-term study presents new data on the nesting behavior of Euglossa melanotricha, obtained from the monitoring of nests found in man-made cavities at a site in northeastern Brazil.
Material and methodsThe present study took place within the urban area of the town of Campo Formoso (10°30'00"S, 40°19'00"W), Bahia, Brazil (
A total of eight Euglossa melanotricha nests were found within the study area, including one inside an electrical installation, and seven in the holes in ceramic building blocks. The nests were transferred to wooden boxes (12 × 10 × 8 cm), each with a circular lateral opening of 1 cm in diameter, according to the methodology developed by
Table 1. Contents of Euglossa melanotricha nests collected in the municipality of Campo Formoso, during the period from August to September 2008 (OC- closed cells; UC – open cells; PC –cells being provisioned; F – females live; F † females dead.
| Nests | Date of transfer | Contents |
|---|---|---|
| N1 | 04/08/2008 | 16 OC; 3 UC; 2 PC; 2 F†; 2 F |
| N2 | 15/08/2008 | 9 OC; 5 UC; 1 PC; 2 F |
| N3 | 02/09/2008 | 11 OC; 1 F |
| N4 | 17/09/2008 | 12 OC; 1 PC; 1 F†; 1 F |
| N5 | 21/09/2008 | 8 OC; 5 UC; 1 PC; 3 F; 4 F† |
| N6 | 24/09/2008 | 8 OC; 1 PC; 1 F |
| N7 | 30/09/2008 | 11 OC; 1 F |
| N8 | 30/09/2008 | 22 OC; 15 UC; 1 PC; 2 F†; 1 F |
The nests were observed under red light between 07:00 h and 18:00 h, with eight hours of observation being conducted three days per week. Nocturnal observations were made once a week between 19:00 and 23:00 h. Quantitative behavioral data were collected using two complementary procedures. All-events records (
Nest development was monitored for the collection of data on the following biological and behavioral parameters: (a) cell architecture; (b) nest reactivation and phenology; (c) female foraging behavior; (d) specific aspects of the activity of the females during construction, i.e. reuse of cells, supply, oviposition, cell closure, oophagy, cleaning the nest and the sealing of edges; (e) duration of the period of offspring development; (f) physiological condition of the females (relative fecundity).
Where appropriate, the results were presented as the mean ± standard deviation. The relationship between the number of cells with eggs and the duration of the female activity period was evaluated using Pearson’s correlation coefficient, while Mann-Whitney’s U was used to test differences in the behavior of dominant and subordinate females, and seasonal variation in the duration of behaviors. Analyses were run in the Statistica 7.0 program, with a 5% significance level.
Results Cell characteristics and arrangementCells of Euglossa melanotricha were elliptical in shape, with a small apical projection.The cells had a mean height of 11.7±0.71 mm (10.8–14.1 mm, n = 97) and a mean diameter of 7.5±0.21 mm (6.2–7.9 mm, n = 97). Females used resin to construct the cells and seal the nests. The cells were generally aggregated in the same plane on vertical or horizontal substrates, either overlapping or not.
Reactivation and nest phenologyThe emergence of 112 females was monitored. Of these, 90 (80.3%) returned to their natal nests, but only 35 (38.9%) remained and actively participated in nest re-use. The other 55 (49.1%) abandoned their natal nests a few days (1–3 days) after their return, without working in the nest. The younger females, more frequently (89.1%, n = 55), abandoned nests days after returning. The great majority (87.1%) of the 31 reactivations observed during the present study involved associations among females. 27 (87.1 %) of these reactivations were performed by more than one female and only four (12.9 %) by one female (Table 2). The reactivation of nests was not synchronized with any specific period of the year. The duration of female activity periods varied from 10 to 78 days (n = 31), and was significantly longer during the rainy season, i.e. between March and August (Table 2: Mann-Whitney, Z = 3.16; p < 0.008, n = 31).
Number of reactivating females and function assumed in the reactivations occurred in Euglossa melanotricha nests, during rainy (March-August) and dry (September-February) seasons.
|
Nest/ Reactivation |
Season | Duration (days) | Number of | ||||
|---|---|---|---|---|---|---|---|
| Rainy | Dry | Associated females | Dominant females |
Subordinate Females |
Oviposited cells | ||
| N1/R1 | X | 54 | 2 | 1 | 1 | 11 | |
| N1/R2 | X | 43 | 2 | 1 | 1 | 9 | |
| N1/R3 | X | 41 | 2 | 1 | 1 | 13 | |
| N1/R4 | X | 28 | 2 | 1 | 1 | 10 | |
| N1/R5 | X | 38 | 2 | 1 | 1 | 13 | |
| N2/R1 | X | 43 | 2 | 1 | 1 | 9 | |
| N2/R2 | X | 52 | 3 | 1 | 2 | 11 | |
| N2/R3 | X | 31 | 2 | 1 | 1 | 10 | |
| N2/R4 | X | 42 | 1 | 1 | 0 | 13 | |
| N2/R5 | X | 22 | 2 | 1 | 1 | 8 | |
| N3/R1 | X | 51 | 1 | 1 | 0 | 10 | |
| N3/R2 | X | 44 | 2 | 1 | 1 | 9 | |
| N4/R1 | X | 53 | 1 | 1 | 0 | 10 | |
| N4/R2 | X | 35 | 2 | 1 | 1 | 12 | |
| N4/R3 | X | 24 | 2 | 1 | 1 | 8 | |
| N4/R4 | X | 37 | 2 | 1 | 1 | 12 | |
| N4/R5 | X | 13 | 2 | 1 | 1 | 5 | |
| N5/R1 | X | 49 | 2 | 1 | 1 | 10 | |
| N5/R2 | X | 10 | 3 | 1 | 2 | 4 | |
| N5/R3 | X | 46 | 2 | 1 | 1 | 10 | |
| N6/R1 | X | 51 | 1 | 1 | 0 | 10 | |
| N6/R2 | X | 23 | 2 | 1 | 1 | 6 | |
| N6/R3 | X | 36 | 2 | 1 | 1 | 12 | |
| N6/R4 | X | 34 | 2 | 1 | 1 | 11 | |
| N6/R5 | X | 26 | 2 | 1 | 1 | 9 | |
| N7/R1 | X | 49 | 2 | 1 | 1 | 10 | |
| N7/R2 | X | 51 | 2 | 1 | 1 | 11 | |
| N8/R1 | X | 78 | 3 | 1 | 2 | 12 | |
| N8/R2 | X | 50 | 3 | 1 | 2 | 13 | |
| N8/R3 | X | 47 | 3 | 1 | 2 | 12 | |
| N8/R4 | X | 38 | 2 | 1 | 1 | 12 | |
During the intervals between reactivations or during periods of inactivity, the females spent more time inside the nests without engaging in cell construction or provisioning. The number of oviposited cells significantly positively correlated with the duration (in days) of the activity period of the females (Table 2: r = 0.6231; p < 0.05, n = 31). The time (in days) spent provisioning cells by subordinate females was significantly longer during the dry season (Wet x Dry season: Z = 3.00; p < 0.001, n = 34).
Social structure and female behaviorForaging behavior: construction or reutilization of cells, provisioning and nectar collection
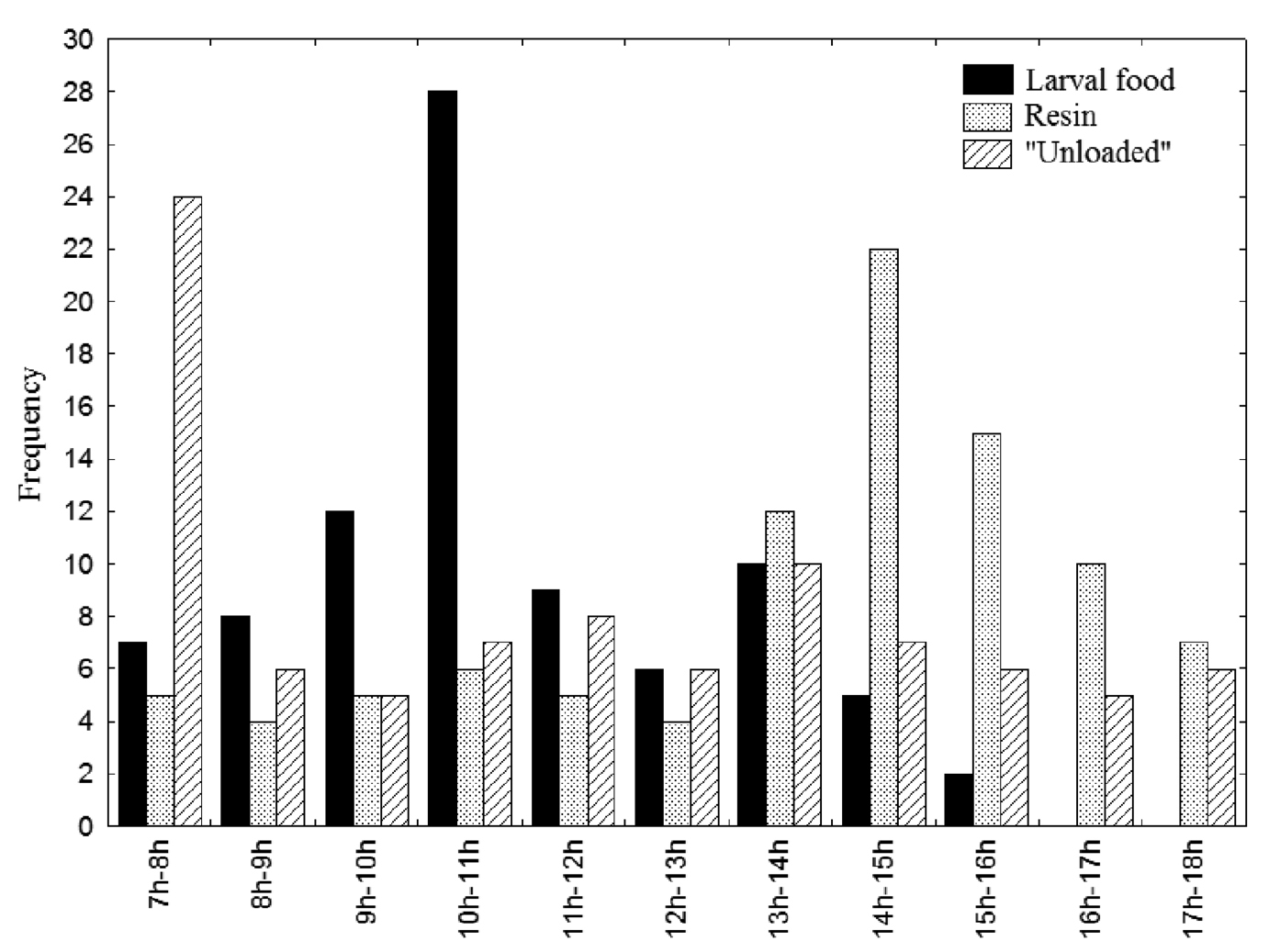
Subordinate females constructed or reutilized cells using resin deposited in small piles inside the nests. Of the 124 cells which were oviposited in, 92 cells (74.2%) were reutilized, while 32 cells (25.8%) were newly constructed. Females began to collect resin two (n = 39) or three (n = 67) days after emergence, and engaged in this activity throughout the day, but with a higher frequency between 14:00 h and 15:00 h (Fig. 1: n = 90). The mean duration of resin-collection trips was 34.3±6.87 min (range: 22–48 min; n = 96).
Frequency of excursion from the nest by subordinate female Euglossa melanotricha according to excursion type (collection of food, resin, or nectar collection “unloaded” ) and the time of day.
Females began to collect and to store food for the larvae four (n = 61) or five days (n = 65) after emergence. The mean duration of food-gathering excursions was 54.4±11.60 min (range: 33–81 min, n = 63), while food storage took 35.1±12.65 s (13–73s, n = 51). Food was gathered primarily in the morning, between 09:00 h and 11:00 h (Fig. 1: n = 99). It took between three and six days for a cell to be provisioned (n = 152).
Females would occasionally return to the nest with neither food nor resin. These excursions were possibly for nectar collection and lasted 27.1±3.58 min (range: 7–68 minutes, n = 78) for dominant females, and 17.5±8.85 min (7–37 min, n = 37) for subordinates. While these trips occurred throughout the day, they were more frequent during the morning (n = 115), primarily between 07:00 h and 08:00 h (Fig. 1).
Specific aspects of the behavior of the females: oviposition and cell operculation, “nest guarding”, cell cleaning, hole sealing and oophagy
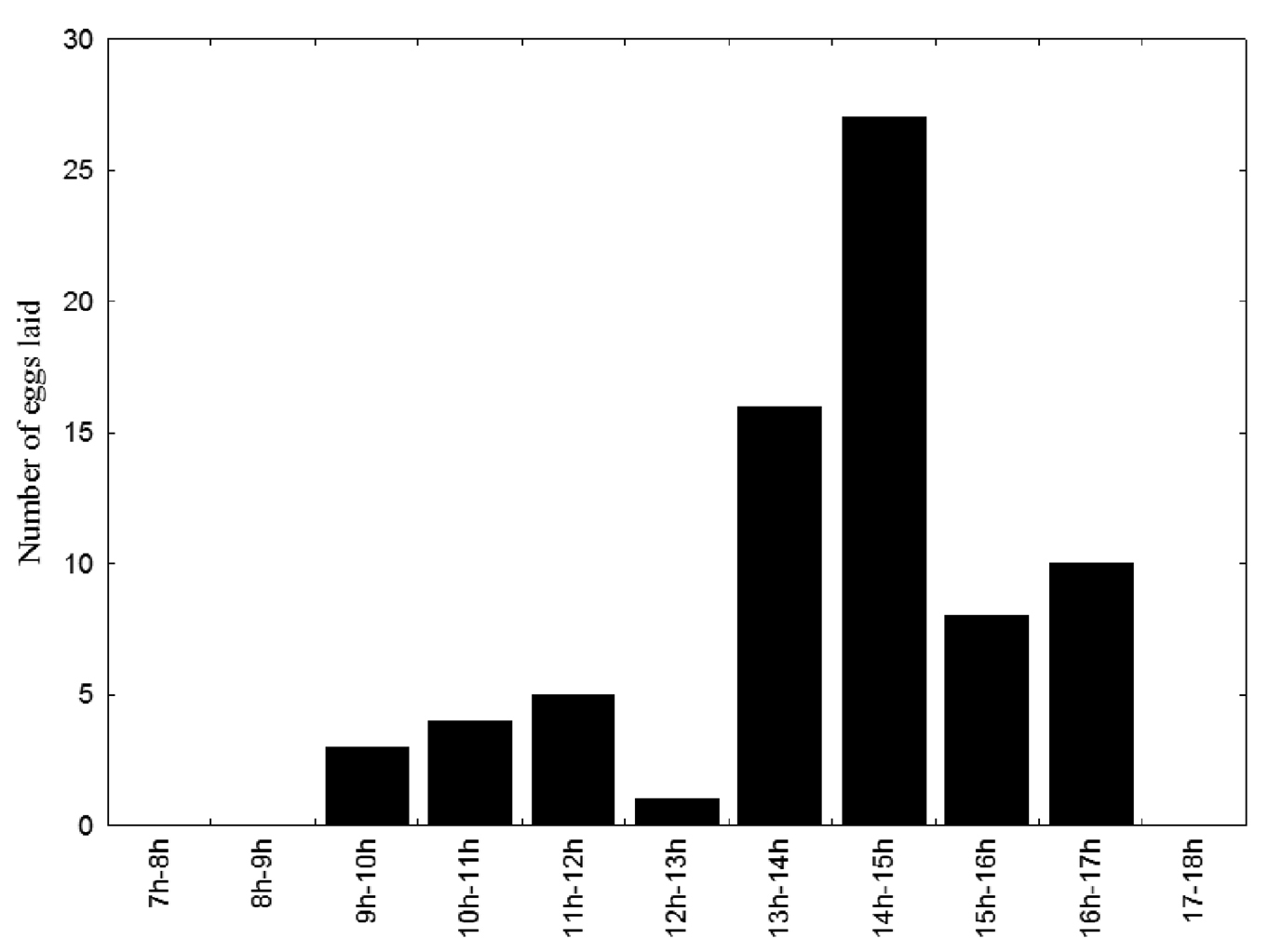
Subordinate females prepared cells for oviposition by building the collar. Construction of the collar took 28.0±13.44 minutes (range: 11–65 min, n = 97) on average. Once they she had built the collar, a subordinate oviposited in the cell, but a dominant female almost always subsequently substituted her egg for that of the subordinate. The duration of bouts of this activity differed significantly (Z = 8.86; p <0.0001, n = 77) between subordinate (120.8±23.48 s, range: 68–155 s, n = 56) and dominant females (88.1±16.70 s, range: 61–125 s, n = 56). Oviposition (n = 97) took place between 09:00 h and 17:00 h, but was most frequent between 13:00 and 15:00 h (Fig. 2).
Frequency of oviposition by female Euglossa melanotricha (dominant and subordinate) at different times of day.
The time spent on cell closure also differed significantly between subordinate and dominant females (Z = 6.08; p < 0.0001, n = 163). While subordinates averaged 16.3±3.47 min (range: 12–24, n = 96) on this behavior, the mean duration for dominant females was 13.0±1.90 min (range: 10–17, n = 67).
Dominant females spent most of their time inside the nests. This behavior was more frequent (70.4%, n = 366) when the subordinates were foraging The average duration of periods spent by dominant females in the nest guarding position was 13.2±6.13 min (range 6–30, n = 366).
Of the 31 processes reactivations observed, in 14 (45.1%) the dominant females disappeared, died or ceased ovipositing and were replaced by another female. In all cases, the substitute was another female that had emerged in the nest.
Following the emergence of a female and before the reutilization of a cell, the subordinate females cleaned the cell by removing the silk and pieces of the cell closure. This detritus was deposited on the bottom of the box. The mean duration of this behavior was 12.0±5.35 min (range: 2–27, n = 306).
The mean duration of resin work bouts was 10.3±5.0 min (range: 2–29, n = 256) for subordinates, and 9.6±4.18 min (range: 2.5–19.8, n = 126) for dominant females. This difference was not significant (Z = 1.04; p > 0.05).
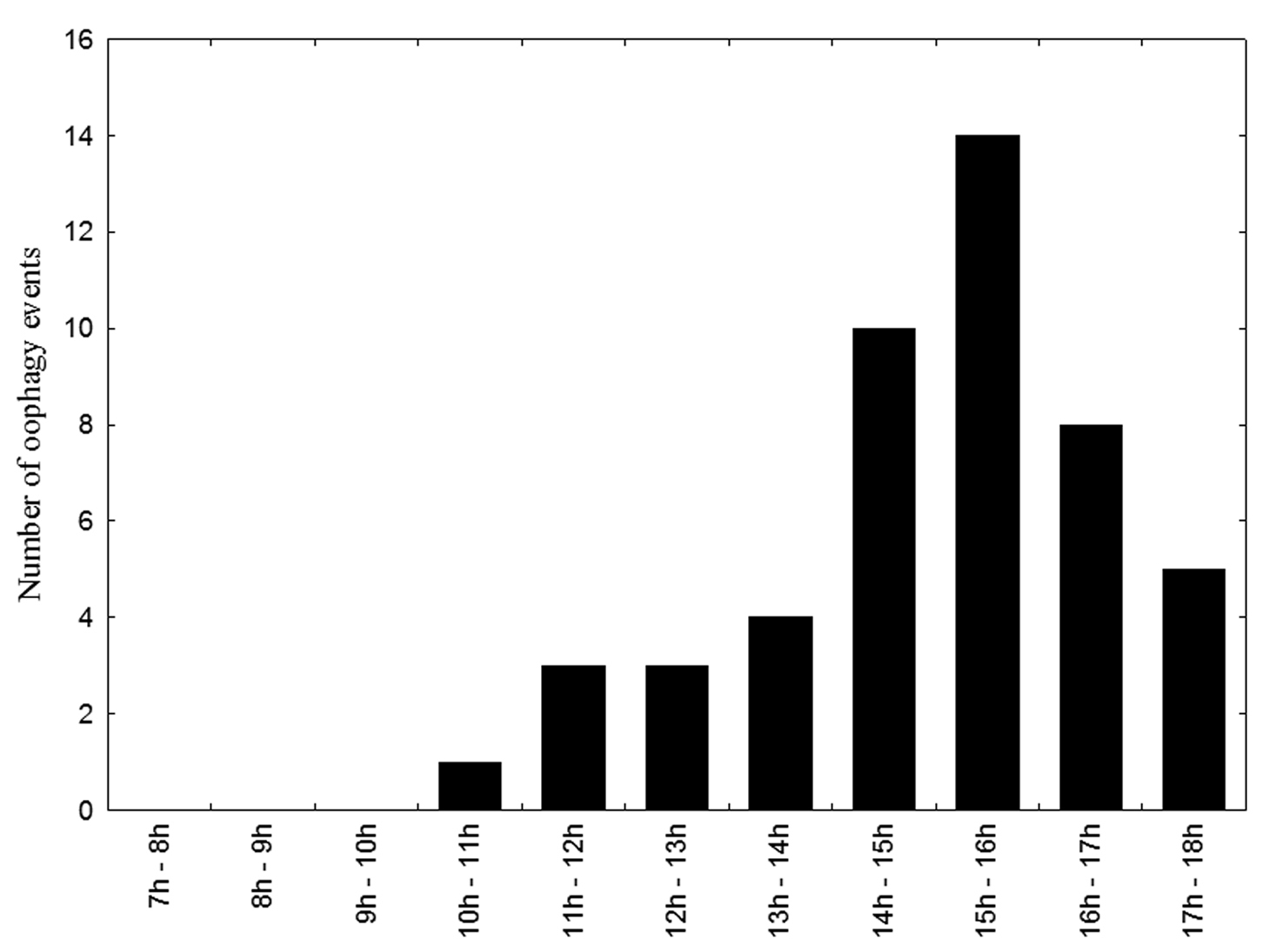
Dominant females opened the closed cells in which subordinates had oviposited after an interval of between 31 and 240.3 min (i.e., more than four hours) after cell closure. Prior to reopening the cells, the dominant females behaved aggressively towards subordinates by biting and pulling them from the closed cell. Opening a cell took an average of 16.7±2.34 min (range: 12.6–21.68 min, n = 62). Following more than half (61% of 141 observed acts) of the subordinate ovipositions, the dominant female ingested the subordinate’s egg. Oophagy took between 96 and 248 s (mean = 158.4±45.65 s, n = 86). Oophagy (86 events) occurred between 10:00h and 18:00h, and was most frequent (n = 61) between 14:00h and 18:00h (Fig. 3).
Frequency of oophagy by dominant female Euglossa melanotricha at different times of day.
Duration of brood development
The length of brood development was compared between the rainy and dry seasons. The period was significantly longer during the rainy season (rainy season – males: 75.7 ± 3.55 days and females: 82.3 ± 1.92 days; dry season – males: 56.2 ± 0.86 days and females: 61.7 ± 2.44 days; Table 3: Z = 4.21; p < 0.0001, n = 26).
Duration in days of the development period (egg-adult) of the brood (male and female) of Euglossa melanotricha during the dry (September-February) and rainy (March-August) seasons.
| Mean development time (egg-adult) in days during the: | ||||
|---|---|---|---|---|
| Dry season | Rainy season | |||
| Nest | Males | Females | Males | Females |
| N1 | 55.2 | 62.3 | 77.6 | 79.5 |
| N2 | 55.6 | 59.3 | 75.4 | 84.3 |
| N3 | 56.1 | 58.9 | - | - |
| N4 | 57.3 | 63.8 | 69.8 | 81.5 |
| N5 | 55.8 | 63.5 | - | - |
| N6 | 57.5 | 58.4 | 76.4 | 82.2 |
| N7 | 55.4 | 64.7 | - | - |
| N8 | 56.6 | 62.4 | 79.1 | 83.8 |
The spermathecae of two dominant and three subordinate females were dissected for the analysis of possible differences related to social rank. The analysis revealed long ovarioles with mature or maturing oocytes and all females were inseminated.
Discussion Cell characteristics and arrangementThe exploitation of pre-existing cavities for the construction of nests observed in Euglossa melanotricha is a behavior typical of most Euglossa species (
The replacement of the dominant female by a subordinate female is consistent with the hypothesis of an age-based dominance hierarchy, as occurs in other primitively eusocial bee species (
The reactivation and abandoning of nests by Euglossa melanotricha followed an asynchronous pattern, which suggests a lack of any systematic relationship with environmental factors. An important aspect of this asynchrony in tropical bees and wasps is the continuous presence of males in the population. This allows the mating of potentially reproductive females throughout the year (
Larval provisioning requires large expenditures of time and energy for Euglossa melanotricha. Besides high costs in time and energy, this amount of time away from the nest could increase the risk of brood parasitization or removal of pollen provisions by scavengers. The presence of parasites Anthrax spp. (Family Bombyliidae) and Hoplostelis bivittata (Megachilidae, Anthidiini) was observed only in nests with a single female in Euglossa viridissima (
Specific aspects of the behavior of the females: oviposition, cell closure, “nest guarding”, cell cleaning, hole sealing and oophagy
In associations of Euglossa carolina, it has been observed that the oldest female assumes nest dominance (
The high rates of return (80%) and effective reactivation (39%) recorded in Euglossa melanotricha were similar to those recorded in Euglossa cordata, Euglossa townsendi, and Euglossa fimbriata (
Because all nest-mates have developed ovaries, have mated, and do not differ in size, dominant and subordinate females are recognized by their behavioral characteristics. Dominant females exhibited agonistic behaviors towards subordinates and the intensities of these aggressive behaviors where the dominant female had already participated in a reactivation process.
The agonistic interactions observed in Euglossa melanotricha can be compared to the behavior of some groups of halictine bees (see
Although the agonistic behaviors displayed by dominant females do not prevent oviposition by subordinate females, reproductive dominance, reflected in the monopolization of offspring production, is achieved by the dominant female through the replacement of subordinate female eggs with her own. The monopolization of offspring production leads to the highest reproductive skew, as predicted by the concession-based transactional skew model (
If the dominant female of Euglossa melanotricha replaces all the eggs laid by subordinates and she mates with only one male, as suggested by
In Euglossa viridissima, no aggressive behavior was observed by dominant female towards her subordinates when they laid an egg, similar to the findings in Euglossa townsendi (
Oophagy of some dominant’s eggs by subordinates was also observed in Euglossa viridissima, however, the dominant cannibalized such eggs and replaced them with her own, confirming her reproductive dominance. The behavior between the dominant and subordinates (associations between mother and daughters) in Euglossa viridissima resembles that of a parasitic female that improves her own fitness on detriment of her daughters’ reproduction (
The oophagy of subordinate’s eggs preceding oviposition by the dominant was also observed in reactivated nests of Euglossa cordata (
As emphasized by Zimmerman et al. (2009), detailed behavioral observations together with the genetic analysis of brood can help clarify the relationships among all females of an association and the real contribution of each one to the social context of the nest.
Oophagy may have a nutritional function (
The females of Euglossa melanotricha sealed the entrance to the nest during the night and when the weather was rainy. This behavior only occurred once all the females had returned from the field. In Lasioglossum (Evylaeus) villosulus, an essentially solitary species which will occasionally associate with conspecifics, the females seal the entrance to the nest in the absence of the other resident females (
Duration of brood development
The development period was similar for males and females, although seasonal variation was influenced by environmental factors, such as the temperature. Higher temperatures may contribute to increased metabolic rates, which may reduce development time considerably (
Presumably single females can establish a new nest. Their subordinate status is determined only by the presence of a dominant.These females may be in a state of “sit and waiting” (
The nesting behavior of Euglossa melanotricha presented in this study provides insights into the social organization of orchid bees. Further studies of the relatedness among individuals will provide data on reproductive partitioning in this species.
We are grateful to the beekeepers Fred and Marcos Rogério for their help with the localization and transfer of the nests, and to Manoel Joaquim and Ana Maria for authorizing access to the nests and to Dr. Stephen Francis Ferrari and two anonymous reviewers for their critical analysis and suggestions for improvement of the text.The Brazilian National Research Council (CNPq) and Fapesp (2010/10027-5) provided graduate stipends to the first author and a productivity grant to F.S.N.