(C) 2012 Bryan Carey. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Eucharitidae is the only family of insects known to specialize as parasitoids of ant brood. Eggs are laid away from the host onto or in plant tissue, and the minute first-instars (planidia) are responsible for gaining access to the host through some form of phoretic attachment to the host ant or possibly through an intermediate host such as thrips. Orasema simulatrix (Eucharitidae: Oraseminae) are shown to deposit their eggs into incisions made on leaves of Chilopsis linearis (Bignoniaceae) in association with extrafloral nectaries (EFN). Nectary condition varies from fluid-filled on the newest leaves, to wet or dry nectaries on older leaves. Filled nectaries were about one third as common as dry nectaries, but were three times as likely to have recent oviposition. Larger numbers of undeveloped eggs, or eggs with mature planidia inside, were associated with filled and wet EFN. For emerged planidia, the distribution was shifted from a concentration at filled nectaries to an even greater concentration at wet nectaries. More planidia were found in EFN (9.50 ±2.85) than outside EFN (1.00 ± 0.60). Planidia were tested for their attachment to adult and larval ants and to adult and immature thrips (potential intermediate host), but the results do not support simple attachment as a viable means for transfer and successful parasitism. Pheidole desertorum was identified as the host ant, and at night is the dominant ant in the tree canopy of Chilopsis linearis. Feeding at the EFN by the host ant, and the direct association with planidia near to or in the EFN, is interpreted as a novel means of accessing the host brood.
Parasitoid, ant, extrafloral nectaries, Chalcidoidea, Formicidae, Pheidole
As with other Eucharitidae, Orasema (Hymenoptera: Eucharitidae: Oraseminae) deposit their eggs away from the host, and the active first-instar larvae (planidia) are responsible for gaining access to the larval ant host (
Females of Orasema oviposit into a variety of plant structures, with eggs deposited into punctures made by the scimitar-shaped ovipositor (cf. Fig. 1a) (
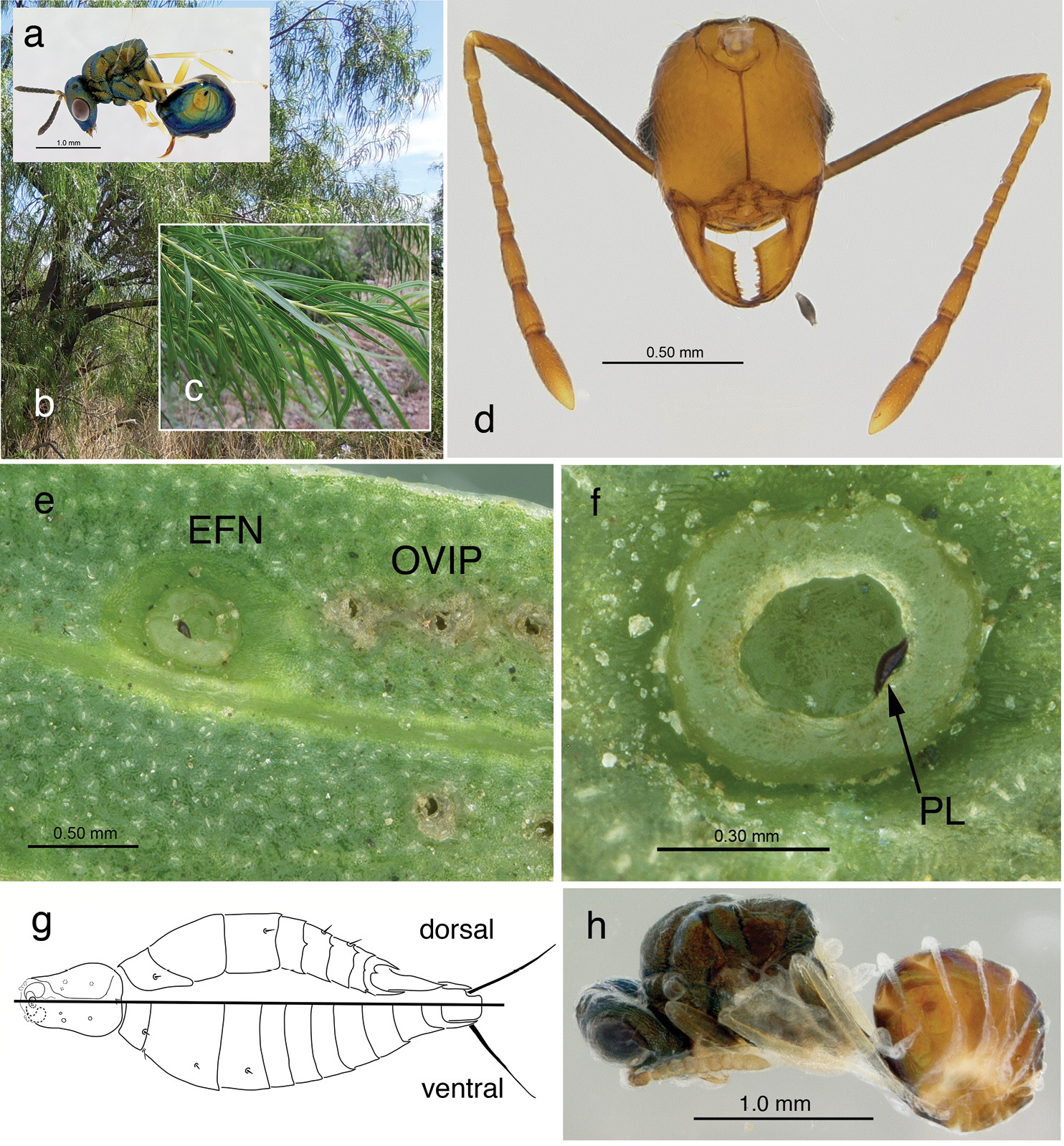
a female of Orasema simulatrix b Chilopsis linearis, habitat c leaf shoot d head of Pheidole desertorum with planidium of Orasema simulatrix e extrafloral nectary (EFN) with planidium inside nectary and oviposition punctures (OVIP) along leaf f EFN with planidium (PL) g planidium with dorsal and ventral view divided by bar h male pupa of Orasema simulatrix.
The Orasema simulatrix group is morphologically distinct and consists of seven species distributed throughout the southwestern US and temperate regions of Mexico (
Bignoniaceae has approximately 100 genera worldwide, but only a few are native to temperate North America (
The simulatrix species group is postulated as being a derived clade among other Nearctic species groups that include species with documented thrips associations (
Geographic Area of Investigation. Data collections, observations, and ant baiting experiments were conducted at four sites near the Southwestern Research Station (SWRS) near Portal, AZ. Ambient temperatures over the year range from night time lows near 5°C in early spring or late autumn to daytime highs of 40°C during summer. Additional sampling and casual observations of Orasema simulatrix on Chilopsis were made at several other localities in California, Texas and New Mexico, at sites ranging from sea level to nearly 2500 meters.
Study Sites. Site 1 (31°52'36"N, 109°03'32"W) was in a stand of six trees (maximum height about 4 m) of Chilopsis linearis in Arizona in the foothills of the Chiricahua Mountains, approximately 8 km east of Foothills Road on Portal Road (leading to San Simon). The habitat was high desert, dominated by sparse trees of Larrea tridentata Coville (Zygophyllaceae), Acacia constricta Benth. (Fabaceae) and Prosopis sp. (Fabaceae), a variety of shrubs including Yucca (Agavaceae), Fouqueria splendens Engelmann (Fouqueriaceae), Opuntia spp. (Cactaceae), and annual herbs in the genera Parthenium, Baileya, Senecio, Gutierrezia (all Asteraceae) and Sphaeralcea (Malvaceae). Site 2 (31°56'14"N, 108°57'59"W) consisted of seven trees in New Mexico in the Peloncillo Mountains, 7 km east of US Highway 80 on New Mexico Highway 9. Site 2 was less vegetated than Site 1, with Larrea tridentata and Chilopsis linearis being the dominant tree species. Shrubs and annuals were also less abundant and diverse. Site 3 (31°55'14"N, 109°42'W) consisted of five trees approximately 0.8 km north of the intersection of Foothills Road and Portal Road. Site 3 was rocky, and the dominant vegetation a mix of Larrea tridentata, Acacia constricta and Prosopis sp. Site 4 (31°54'50"N, 109°07'42"W) consisted of 5 trees and began at the intersection of the above-mentioned roads, and extended south and east along Portal Road. The trees were spread over a distance of 0.25 km alongside the road, within 50m of the road edge. The dominant tree species were the same as for Site 3. In 1999, Sites 1 and 2 were abandoned for study because of low activity of Orasema simulatrix. At Site 2, the area was in extreme drought, and no wasps were recovered. Site 1 was abandoned because the Arizona Department of Highways chose to improve the shoulders of Portal Road and leveled the entire stand of Chilopsis linearis. Therefore, in 1999, studies were shifted to Sites 3 and 4, which were approximately 1 km apart. Sites 3 and 4 were revisited in September 2011 for additional collections of leaves, immature stages of eucharitids, and foraging ants.
Insect and Plant Identifications. Museum collections examined were University of California, Riverside, CA (UCRC), Texas A&M University, College Station, TX (TAMU), University of Arizona, Tucson, AZ (UAZC), and the Southwestern Research Station, Portal, AZ (SWRS). Voucher specimens of wasps, ants and thrips were deposited in the UCR Entomology Research Museum (UCRC) (voucher code BC1). Ants were determined or verified by S. Cover (Museum of Comparative Zoology) or R. Snelling (Los Angeles County Museum). Slide mounted thrips were identified by L. Mound (Commonwealth Scientific and Industrial Research Organization, Australia). Host plant vouchers were deposited in the UCRC Herbarium, with identifications provided by A. Sanders (UCRC).
TerminologyHost Plant. Terminal growing shoots were used for sampling, with a growing shoot defined as any single branch without subtending shoot growth and having at least 20 leaves and some extrafloral nectaries (EFN). Typically, leaves at the tip are less than 4 cm long, while mature leaves are about 10 cm long (Fig. 1c). Shoot-tip leaves can have EFN, but these are usually inaccessible to wasps because they are grouped in a tight cluster. Three qualitative conditions of EFN were identified: “filled” when the nectary had a distinct convex meniscus of fluid, “wet” when the quantity of fluid did not equal or exceed the perimeter of the nectary, and “dry” when it contained no obvious fluid. EFN are reabsorbed on older leaves, and oviposition activity was not observed on older shoots that lacked any nectaries. Examination of shoots included up to 60 leaves from, but not including, the clustered shoot-tip leaves. Leaves were grouped into categories of ten consecutive leaves, with Category I containing the youngest leaves, 1-10, and so on through leaf 60. This allowed for the number and condition of EFN to be correlated with the relative age of the growing shoot. EFN are also found on the subtending bracts of flowers and sepals; these were also examined for oviposition, but these were rare events.
Immature Orasema. Immature stages of Orasema observed on leaves were categorized as undeveloped eggs, developed eggs, and planidia. Undeveloped eggs were white in color, indicative of recent oviposition. Developed eggs were dark brown to black in color, with the developing planidium visible inside the egg. Emerged first-instar larvae (planidia) were dark brown to black and could be seen crawling, maggot-like, along plant parts or congregated in nectaries.
Oviposition habits of OrasemaThe distribution of oviposition punctures on plant structures of Chilopsis linearis did not appear to be random, so a series of experiments was designed to test for a preference for plant structure, concentration of oviposition on particular structures, and choice along shoots.
Oviposition Pattern and Abundance. In 1998, each site was sampled three times (July 30, August 20, August 27). Five shoots were removed from each of two trees and returned to SWRS for analysis. Samples were stored in paper sacs within Ziploc ® plastic bags and refrigerated at 4ºC. Each leaf or flower was removed sequentially from the apex of the shoot to the base and examined using a Leica Wild M5 stereomicroscope for presence of nectaries and distribution of oviposition punctures. Measurements of nectary size and the maximum distance of punctures from the associated nectary were made using an ocular micrometer (0.01 mm divisions). Females of Orasema deposit single eggs into a linear series of punctures formed in the plant tissue by the ovipositor (
Oviposition choice. Sampling was conducted to determine preference for oviposition along the branch or next to nectaries. In 1999, five trees at Site 3 and 4 were sampled sequentially from July 7–13 and from July 18–30, respectively. Two shoots were sampled per day from one tree at each site and processed within 18 hours of removal. Samples were processed in a similar manner to the 1998 sampling. Counts focused on the condition of nectaries and immature stages (eggs and planidia) along shoots. Nectaries were assessed for quality (filled, wet or dry) and eggs within each puncture were assessed for condition (undeveloped, developed or emerged) by dissecting oviposition punctures using a number 3 insect pin. The condition of eggs within a series could not always be accurately identified because of difficulties with dissection of an egg from a puncture. Because all eggs within a series are deposited at a single oviposition, the condition of any egg within a series was considered representative of the entire series. Therefore, the number of eggs is an estimate, not necessarily an accurate count of the condition of each individual egg. Counts were also made of the number of planidia crawling free on the leaf surface or within nectaries. The distributions of EFN and of immature stages were compared by partitioning leaves into categories as described above. The distribution of oviposition sites among leaves or EFN was compared with a Chi square test for homogeneity (using Excel 1997). Differences in the distribution of undeveloped eggs and planidia at filled, wet, and dry nectaries were analyzed by Kruskall-Wallis rank test using Statview 4.02 (
Regrowth can occur after damage (e.g., by grazing ruminants) and can result in a large flush of new growth. Regrowth branches are fundamentally different in that filled nectaries are distributed over the entire length, rather than concentrated at the anterior end of a shoot. In two samples, this resulted in an apparent oviposition bias, and these samples were excluded.
Ant AssociationsEarlier discussion focused on interactions between Orasema and Chilopsis linearis. We were interested in the distribution of potential ant hosts on the plant and foraging activities that might place them into direct contact with planidia of Orasema simulatrix. Investigations consisted of nest excavations of potential ant hosts in the near vicinity of wasp-infested Chilopsis (based on assessment of foraging activity to baits on the ground and tree), and observations of arboreal foraging in the absence of baits.
Host Record. Broods of several species of ants were excavated in the vicinity of infested Chilopsis at the four study sites. Numerous excavations also were made at other locations; these results are not reported but were similar to the results from the study sites. Brood and workers were collected and preserved in 75% EtOH and then examined for parasitism using a Leica Wild M5 stereomicroscope. Excavated soil containing brood from nests of Solenopsis or Pheidole was placed into Fluon-lined plastic containers (60 cm × 50 cm). Over two days, brood, ants, and any parasites were sorted out of the soil with forceps, a hand trowel, thin paintbrushes, and an aspirator. Brood was examined for parasitoids, and then either preserved in EtOH or maintained in a live colony.
Live colonies were maintained in artificial humidity chambers made of a plaster-filled plastic petri dish (90 mm × 15 mm) with three 3 mm holes drilled laterally and one 3 mm hole in the lid of the dish as entrances. Each chamber was covered by red plastic film to act as a light filter. Chambers were placed into larger polyethylene containers (various sizes) in which the sides were coated with Fluon to prevent ants from escaping. Water was offered in cotton-stoppered glass vials. Food consisted of peanut butter and sugar water offered continuously and freshly killed insects occasionally.
Host Foraging. In addition to investigations of ant nest distribution, observations were conducted in 1998 to determine the foraging patterns of ants near or on Chilopsis linearis infested with Orasema simulatrix. The expectation was that one or more myrmicine ant species, such as Pheidole desertorum, would be present and foraging. Ant distribution was characterized either by infrequent monitoring, or using peanut butter bait stations that preferentially attract Myrmicinae (
From 1997–1999, tests were conducted to determine if first-instar larvae (planidia) of Orasema simulatrix will attach to adult ants, larval ants or immature thrips. Using a minuten pin, insect pin, or thin paintbrush, individual planidia were placed into clear plastic tubes (ca. 10 mm × 5 mm), with each end of the tube plugged with cotton. After insects were introduced, the tubes were taped to index cards and placed into a closed polyethylene container (132 cm × 21.5 cm × 22 cm) over a saturated salt solution to maintain a relative humidity of 75%, and in a growth chamber at 21°C and 14 L:10 D photoperiod cycle. Two types of associations were tested. In a non-contact association, a planidium was placed into the vial first, and an associate (thrips, immature ant or adult) introduced nearby within the vial, but not contacting the planidium. In a contact association, a planidium was placed directly onto the associate. For each trial, the planidium and associate were checked intermittently over a 36 hour period, or until one of the two insects died. At each observation, planidia were monitored as to whether they were unattached, attached externally, or if they had burrowed into the associate.
Combinations with the planidia placed near, but not on the host, were conducted only with immature thrips (n = 12). Combinations with planidia placed onto the insect associate were: a) planidia on immature thrips (n = 45), b) planidia on mature ant larvae (Solenopsis n = 8, Pheidole desertorum n = 8), c) planidia on an adult worker (Solenopsis n = 12, Pheidole desertorum n = 10, Pheidole tucsonica n = 8). Individual ant workers were also combined with sections of leaf that included a single nectary with one or more planidia within the nectary (n = 12).
In 2011, 250 workers of Pheidole desertorum were sampled by either sweeping or aspirating Chilopsis linearis at night over a 6 hour period. Numerous planidia were present both on the leaves and within EFN on the trees sampled. Foraging ants were examined both externally and internally (mouthparts) for planidia.
Results Collections and Host RangeOrasema simulatrix has been collected from numerous localities in southern California, Arizona, New Mexico and western Texas (locality information available on request). Museum records include collections from several different plant families, including Apocynaceae, Asteraceae, Bignoniaceae, Fabaceae and Malvaceae. This apparent diversity is likely misleading, as most records for Orasema simulatrix are from only one or two specimens collected for each plant species. All of these records are from random sweep-net samples, and no cases of oviposition were recorded. Although different angiosperms were sampled as part of this study, only two specimens of Orasema simulatrix, one male and one female, were ever sampled from another plant, Acacia constricta Benth. (Fabaceae) (Site 4). Oviposition was observed only on Chilopsis linearis.
Oviposition ChoiceUsingtheir scimitar-shaped ovipositor, females of Orasema simulatrix oviposit into leaves of Chilopsis linearis, with the single egginserted parallel to the leaf surface. Females oviposit in a linear series of 1–27 eggs, with an average of 3.27 eggs per series. Females preferentially deposited their eggs into leaves with extrafloral nectaries (Table 1). Of 310 leaves sampled in 1998, 57% (176) had one or more EFN, and 76% of these (134) had oviposition punctures (Table 1). Only three of the 134 leaves without EFN (2%) had oviposition punctures. Leaves with EFN had a total of 509 extrafloral nectaries, averaging 2.89 ± 1.41 (SE) EFN per leaf. There were a total of 2599 punctures, of which 2546 occurred on leaves with EFN. Of 798 leaves sampled in 1999, a smaller proportion of leaves (327; 41%) had EFN, but these included the majority of leaves with oviposition punctures (268) and a total of 5, 790 punctures (Table 1). There was a marked difference between sites, as Site 3 had 1, 940 punctures next to 270 EFN on leaves and Site 4 had 4, 151 punctures next to 381 EFN on leaves. Only 12 of the 471 leaves without EFN (2%) had oviposition. In contrast to leaves, 47 flowers contained only 13 oviposition punctures (Table 1). Extrafloral nectaries (EFN) were found on both bracts and sepals. Eggs deposited next to EFN on bracts or sepals were placed in close proximity to EFN, as they were on leaves, although because of the small size of the bracts and sepals, it is possible that eggs were deposited randomly.
Distribution of oviposition punctures on Chilopsis linearis for 1998/1999. No flowers (sepals or floral bracts) were sampled in 1998.
| Leaves with EFN | Leaves without EFN | Sepals | Floral bracts | |
|---|---|---|---|---|
| number examined | 176 / 327 | 134 / 471 | – / 47 | – / 113 |
| number with punctures | 134 / 268 | 3 / 12 | – / 2 | – / 3 |
| total number of punctures | 2546 / 5790 | 53 / 301 | – / 4 | – / 9 |
| % with punctures | 76 / 82 | 2 / 3 | – / 4 | – / 3 |
Females deposited eggs in close association with the EFN. The average distance of oviposition marks from the edge of an EFN was 2.38 ± 0.20 mm based on 14 shoots, 61 leaves, and 140 nectaries. Leaves were approximately 10 cm long, and the average nectary diameter was 0.26 ± 0.01 mm. Oviposition marks could be on both sides of the leaf. However, the number of adaxial oviposition punctures per leaf was 1.05 ± 0.34, as compared to 7.15 ± 1.09 abaxial ovipositions per leaf. The average number of ovipositions per EFN on the same side of the leaf midrib as the EFN was 5.88 ± 0.42, with 4.67 ± 0.35 across the midrib from the nearest EFN.
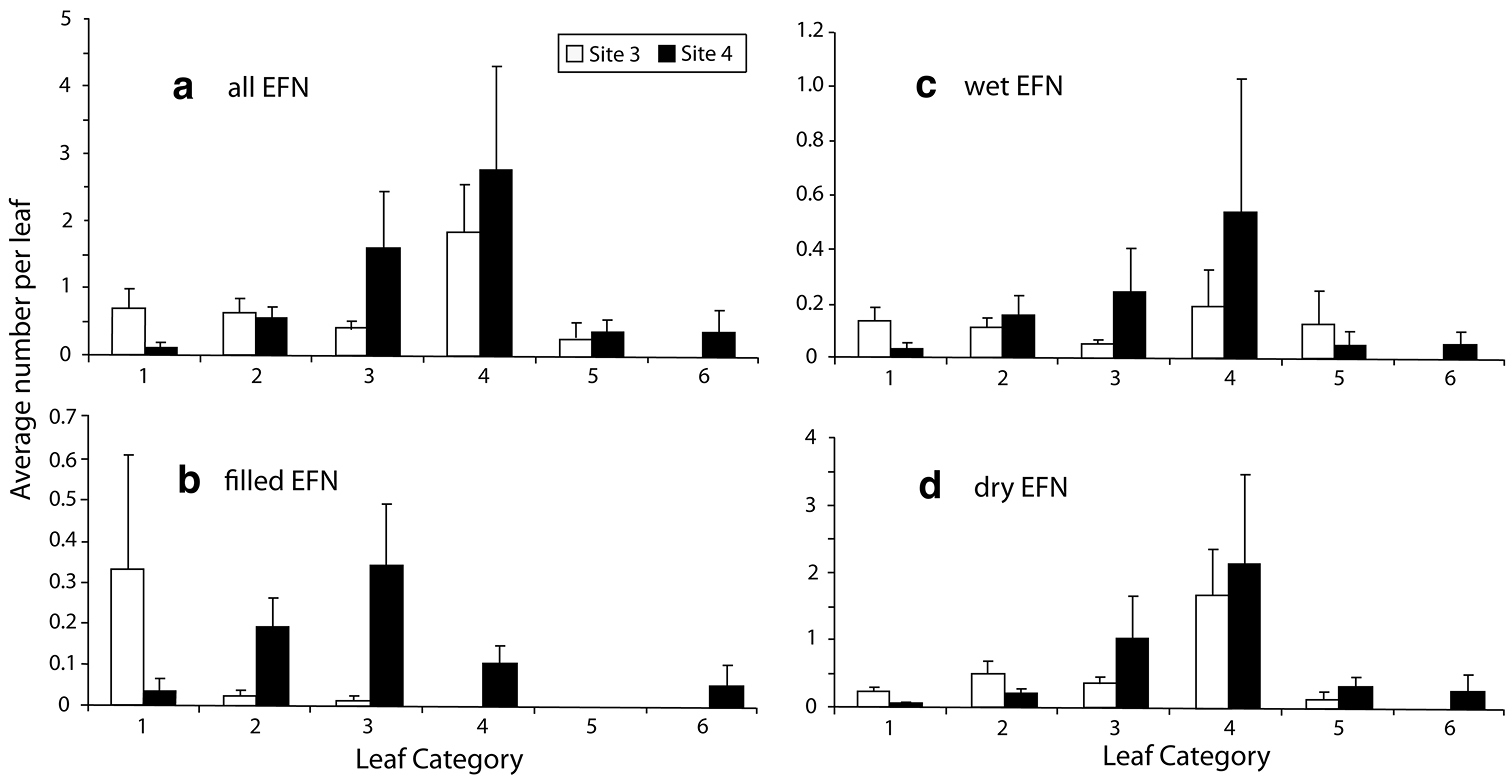
Distribution of Nectaries and Immatures along ShootsDistribution of nectaries.Chilopsis linearis shoots averaged 39.9 leaves for the twenty shoots sampled. Because of differences in shoot length, not all shoots had a full complement of 10 leaves in the more basal categories (4, 5 and 6). The distribution of nectaries along shoots of Chilopsis linearis was not uniform across leaf categories (Fig. 2). Nectaries were most abundant on leaves in Category 4 (leaves 31-40 distal to branch tip), with an average of 2.37 ± 1.90 nectaries (Fig. 2a). Most nectaries were dry (Fig. 2d), suggesting they were no longer a potential food source for ants. For Category 4 leaves, the proportion of wet nectaries was much greater than filled, but much less than the dry or senescing nectaries. The number of nectaries per leaf declined dramatically in Categories 5 and 6 (Figs 2a–d). This decline is probably a result of reabsorption of the nectaries on these older leaves, although it could also represent fewer nectaries present on these leaves when initially formed.
Distribution of extrafloral nectaries (EFN) on shoots of Chilopsis linearis in AZ, 1999. Shoot defined as branch with new growth and leaves with EFN. a all EFN b filled EFN c wet EFN d dry EFN.
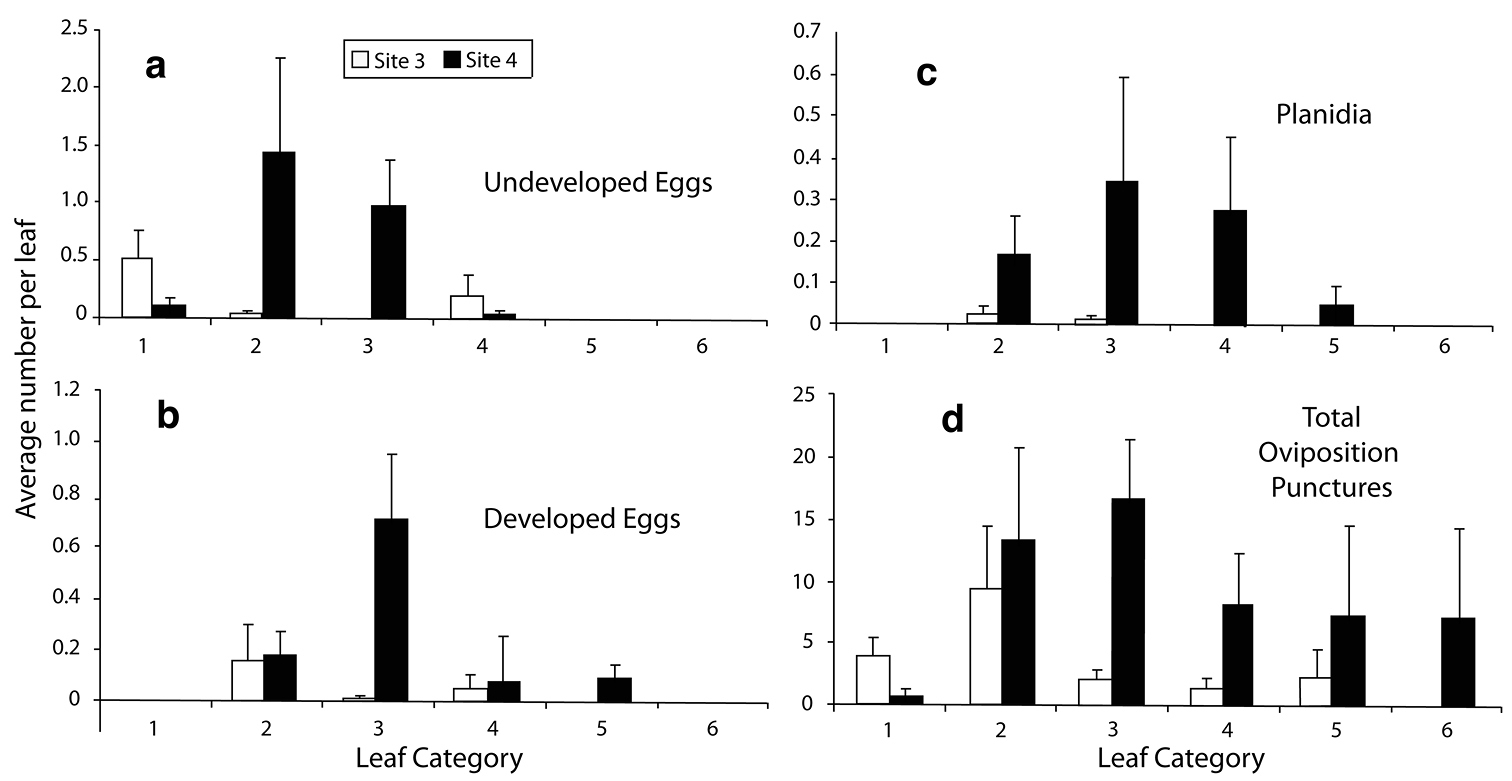
Distribution of Immatures. The condition of eggs was used to gauge the relative time since oviposition by females, with white or undeveloped eggs indicating recent oviposition, and dark or developed eggs indicating earlier oviposition. Assuming that planidia did not wander far from the oviposition site, especially to different leaves or different leaf groups, their presence corresponds with an even earlier oviposition event. Empty punctures could be the result of previous cohorts of Orasema and were not used as indicators of activity. The distribution of immature stages was not uniform for the typical growth shoots and there was a significant difference in the distribution of immature stages at the two sites (X2 = 5.59) (Fig. 3).
a–c Distribution of immature stages of Orasema simulatrix among leaf categories on Chilopsis linearis in AZ, 1999 d total number of oviposition punctures on Chilopsis linearis. Regrowth samples excluded (see text for details).
There was a progression from younger to older immature stages from the apical leaf groups to the more basal leaf groups (Fig. 3a-c). The distribution of immatures was very different between sites, likely representing different peaks of activity, with Site 1 having both fewer eggs, and a much higher proportion of empty (emerged) punctures (Fig. 3a–d). The number of undeveloped eggs varied from 0.51 ± 0.25 in Category 1 for Site 3 to 1.44 ± 0.82 in Category 2 for Site 4 (Fig. 3a). No undeveloped eggs were found on the older Category 5 and 6 leaves at either site. Developed eggs (planidia ready to emerge) were found only on leaves from Categories 2 through 5, and most numerous in Category 2 for Site 3, with 0.15 ± 0.14 eggs, and in Category 3 for Site 4, with 0.71 ± 0.38 (Fig. 3b). Planidia were not found in the Category 1 leaves at either site. Planidia were mostly distributed medially on a shoot, with the highest number in leaf Category 3 for Site 4 (0.34 ± 0.25), while planidia were scarce, but highest in Category 2 for Site 3 (0.02 ± 0.02; Fig. 3c). The distribution among immature stages was not correlated with the distribution of total number of nectaries along the branch; however, the distribution of undeveloped eggs (most recent ovipositon events) could be roughly correlated with the larger number of filled and wet nectaries in Categories 1 to 3. More undeveloped eggs were found to be associated with filled nectaries than wet nectaries, although neither distribution was significant (Spearman Rank correlation coefficient P = 0.586 and p = 0.19 and P = 0.757 and p = 0.09, respectively).
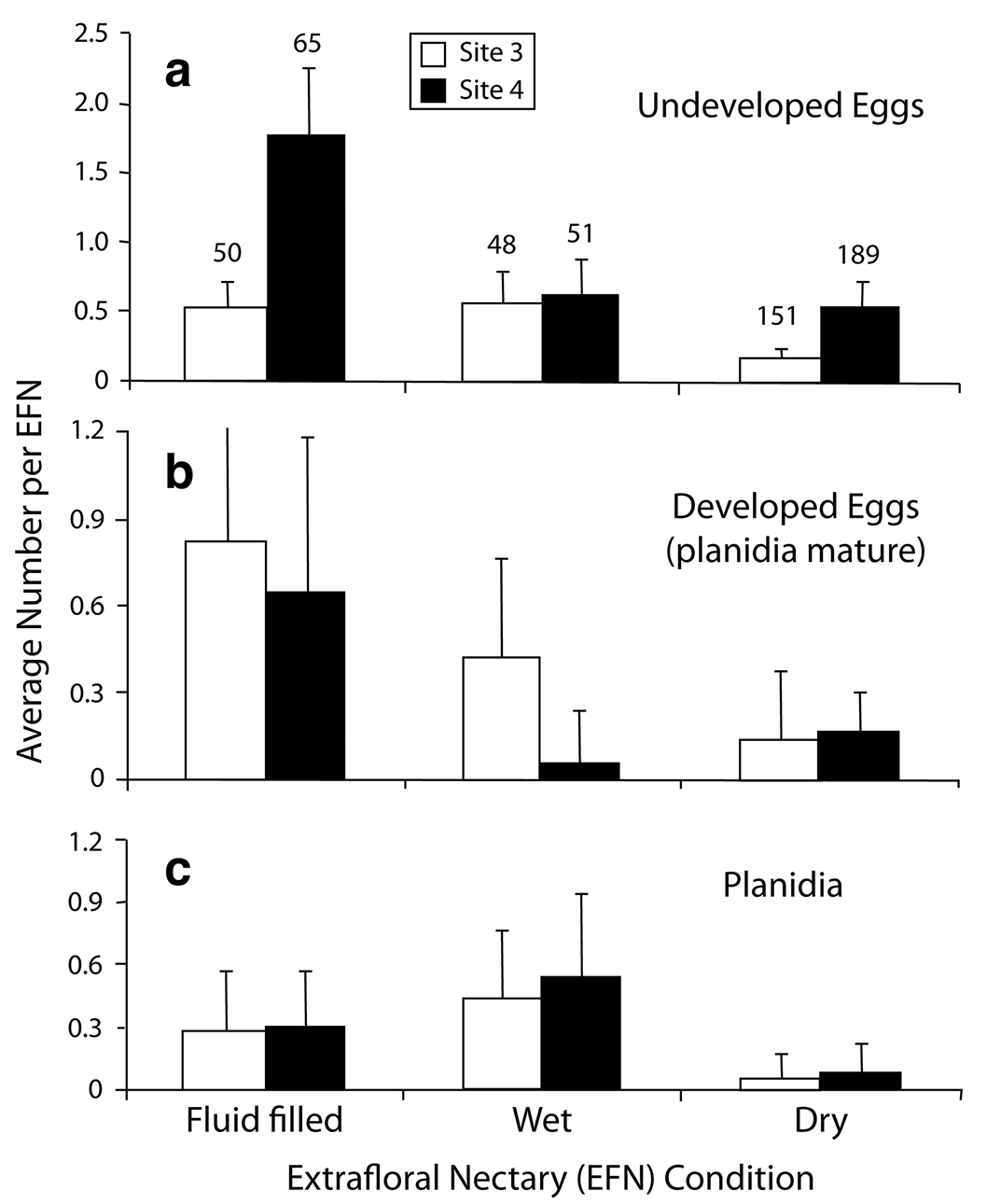
Association by EFN type.Overall, 85% of EFN had associated oviposition marks (554 of 651). The greatest number of undeveloped eggs per EFN (1.78 ± 0.48) was associated with fluid-filled nectaries at Site 4 (Fig. 4a). The least number of undeveloped eggs were associated with dry nectaries, even though dry nectaries were numerically more abundant at sites 3 and 4 (Fig. 3). Although filled nectaries were about one third as common as dry nectaries, they were three times as likely to have undeveloped eggs indicating recent oviposition. Larger numbers of the undeveloped eggs were associated with filled and wet EFN; this pattern was significant at Site 4 (Kruskall-Wallis H2 = 30.65, p = 0.0001), but not Site 3 (Kruskall-Wallis H2 = 4.98, p = 0.08). Developed eggs followed a similar pattern to that of undeveloped eggs, with the greatest concentration at filled EFN, with 0.82 ± 0.86 and 0.65 ± 0.54 for Site 3 and 4, respectively (Fig. 4b). For planidia, the distribution was shifted from a concentration at filled nectaries to an even greater concentration at wet nectaries (Fig. 4c). The three EFN conditions were tested for differences in number of planidia and were significantly different at Site 1 (Kruskall-Wallis H2 = 9.891; p = 0.0071). For both sites, more planidia were in the EFN (9.50 ±2.85) than outside EFN (1.00 ± 0.60). Additionally, planidia were most uncommon at wet nectaries at Site 4 (0.06 ± 0.17).
Distribution of immature stages among the three conditions of extrafloral nectaries (EFN) in AZ, 1999. a undeveloped eggs b developed eggs with mature planidia c planidia. Regrowth samples excluded.
Host Record. A single nest of Pheidole desertorum was excavated that contained larvae and pupae of Orasema simulatrix (Fig. 1h). Of 30 excavated nests of various ant species nesting near Chilopsis linearis, only one Pheidole desertorum nest contained Orasema simulatrix (Table 2). This is the first host record for any member of the Orasema simulatrix species group. Of 229 ant larvae, 97 (42%) had a total of 119 planidia. Planidia were either surface-attached or embedded (c.f.
Ant nests excavated within two metres of Chilopsis linearis (1997–1999).
| Ant Species | Colonies with Brood | Colonies without Brood | Number of Nests Parasitized |
|---|---|---|---|
| Aphaenogaster cockerelli Andre | 1 | 0 | 0 |
| Camponotus festinatus Buckley | 0 | 3 | 0 |
| Crematogaster sp. | 0 | 1 | 0 |
| Forelius pruinosus Roger | 1 | 0 | 0 |
| Formica sp. | 1 | 4 | 0 |
| Monomorium sp. | 1 | 1 | 0 |
| Myrmecocystus mimicus Wheeler | 0 | 3 | 0 |
| Pheidole desertorum Wheeler | 2 | 2 | 1 |
| Pheidole tusconica Wheeler | 1 | 1 | 0 |
| Solenopsis aurea Wheeler | 1 | 1 | 0 |
| Solenopsis xyloni McCook | 2 | 2 | 0 |
| Tetramorium hispidum Wheeler | 1 | 1 | 0 |
Morphology of Immature Stages of Orasema. Egg. The undeveloped eggs of Orasema simulatrix, as found in plant tissue, are white, elliptical in shape, and stalked, as described for nearly all eucharitids (
Ant Presence and Activity. Twelve species of ants were found nesting within two meters of Chilopsis linearis from which adult Orasema simulatrix had been swept (Table 2). Of these, Pheidole (2 spp.) and Solenopsis (2 spp.) are known hosts for Orasema (
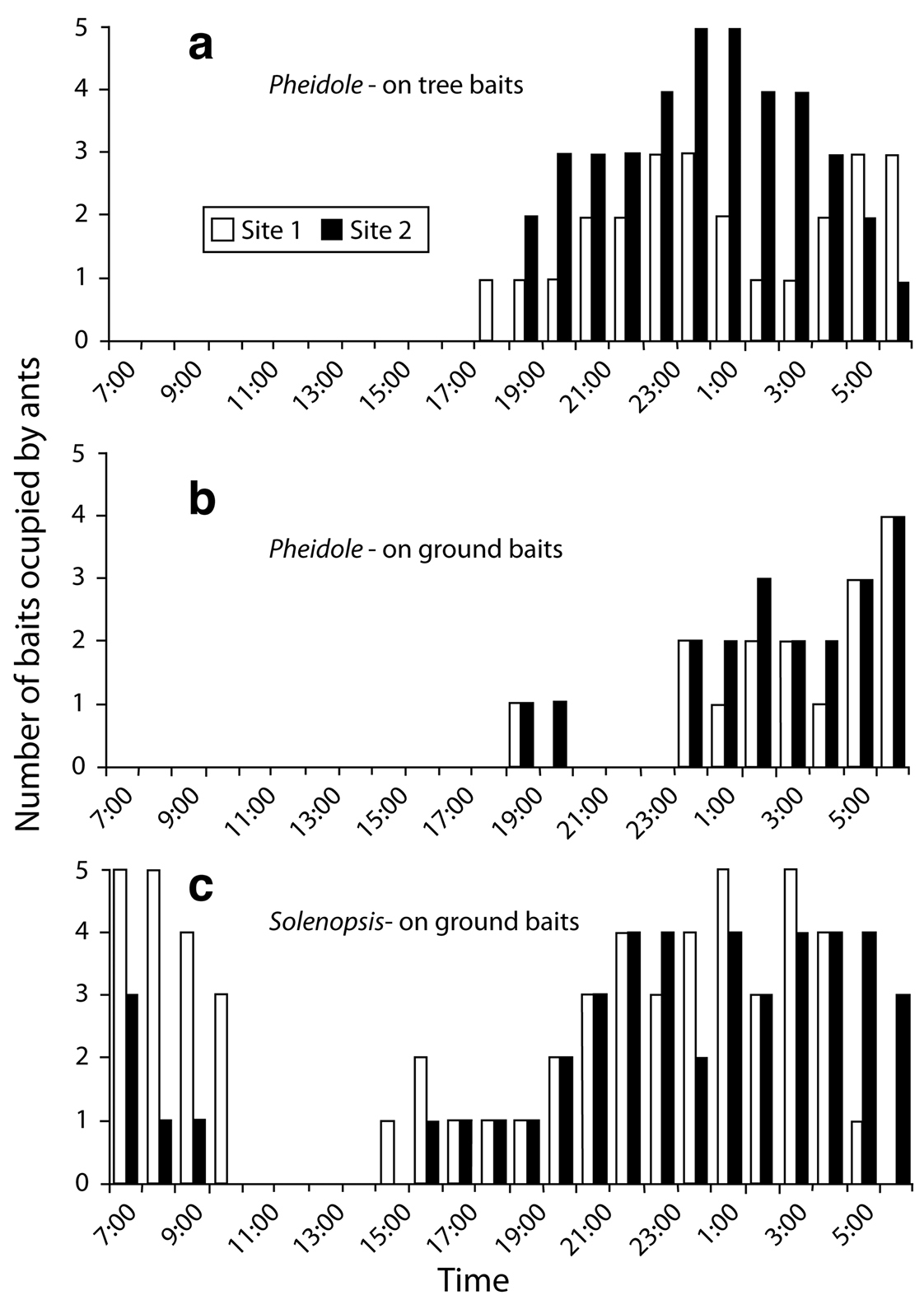
Camponotus festinatus (Buckley), Myrmecocystus mimicus and Pheidole desertorum were nocturnal species that foraged at and drank from extrafloral nectaries on Chilopsis linearis. Similar foraging and drinking activities at the nectaries were observed for diurnal ants such as Forelius pruinosus (Roger) (commonly, but in low numbers) and Monomorium (rarely and only a few individuals). Forelius was commonly sampled at bait stations, but the other diurnal ants were only rarely sampled at baits (less than 15 individuals in all studies). Pheidole desertorum was the most commonly seen ant in baits in the tree at night (Fig. 5a). Pheidole tucsonica Wheelerand Solenopsis sp. were not observed at baits in the canopy of Chilopsis linearis. Pheidole desertorum was common at ground bait stations (Fig. 5b), but was largely superseded by Solenopsis xyloni (Fig. 5c). Foraging activity by Pheidole was similar at both sites and time periods sampled, beginning close to sunset (6:00 P.M.) and ending by sunrise (7:00 A.M.) with peak activity through the night. Solenopsis xyloni had a much longer period of foraging, being present at 3:00 P.M. through 11:00 A.M. the following morning. At the ground bait station, both Pheidole desertorum and Solenopsis xyloni could be found together, but only when there were less than 10 individuals of either species. Besides the dominant, ground-foraging Solenopsis xyloni, other species that were moderately active at baits on ground included other myrmicines such as Aphaenogaster cockerelli, Pogonomyrmex rugosus, and Pogonomyrmex barbatus, and several non-myrmicines such as Formica, Myrmecocystus and Forelius. Rarely observed ant species that foraged at ground baits included Tetramorium hispidum, Crematogaster sp., and Pheidole tucsonica.
Ant abundance at baits on or near Chilopsis linearis in AZ, 1998. a Pheidole desertorum on tree b Pheidole desertorum on ground c Solenopsis xyloni on ground. Only Pheidole desertorum was recovered at baits in the tree.
Thrips and Ant Interactions. Neohydatothrips sp. (Thripidae) was the only thrips found associated with Chilopsis linearis. This is a polyphagous, flower-loving genus, found in the Nearctic and widely distributed in the Neotropics (L. Mound, pers. comm.). Immature and adult thrips were observed mostly in flowers or on leaves in the terminal growing tip of branches of Chilopsis linearis, but only rarely were adults or immatures found on mature leaves (Categories 1–6), and none were observed in direct association with leaf nectaries. None of the adult thrips were observed to harbor planidia. Of 143 immature thrips observed from field samples (all plant structures), none had an attached planidium.
To explore the interaction between planidia and immature thrips, single planidium were placed close to but not on a single immature thrips (12 trials), and directly on the immature thrips (45 trials). After at least four hours, only 4% (2/45) of the planidia remained on the immature thrips. Of these, one planidium remained attached to the surface of the thrips, while the other burrowed through the cuticle of the immature thrips as has been reported for other species of Orasema (
Other experiments with Orasema simulatrix investigated several behavioral components of the planidia, including the attachment of planidia to adults of three ant species. One of eight planidia remained on workers of Pheidole tucsonica. Of 10 planidia placed on workers of Pheidole desertorum, none remained attached after a similar time period. Two of 12 planidia remained attached to workers of Solenopsis xyloni. Thus, only three of 30 planidia (10%) placed on workers remained attached after four hours. These planidia remained where they were placed on the ant host and did not migrate to what might be considered as appropriate places for transfer to the larval host, such as the mouthparts. Planidia were placed on eight mature larvae of Pheidole desertorum and eight mature larvae of Solenopsis xyloni; no planidia remained on the host for more than four hours. In 1999, 83 foraging ant workers were collected from leaves and branches of Chilopsis linearis at Sites 3 and 4; none had attached planidia. Twelve trials were set up in which one to five ants were placed in petri dishes with leaves of Chilopsis that had filled or wet nectaries and planidia. Ants did not show any particular interest in the nectaries, possibly as a result of the artificial setup, and overnight, no planidia were found attached to the included ant workers. Ants offered thrips with and without attached planidia were not observed to prey on or carry thrips alone, or thrips with planidia placed on their bodies. Of 250 adult workers of Pheidole desertorum collected in 2011 from Chilopsis linearis (near Site 3) infested with planidia of Orasema simulatrix, none of the workers had planidia attached externally or, after dissection, embedded within their mouthparts. Thus, to date no direct observations have been made of the planidia being transferred by the ant host to the nest.
DiscussionPlanidia of several species of Orasema representing most New World species groups and one Old World species group are postulated as being dependent on an interaction with intermediate hosts to move from their plant hosts to the nest of their ant hosts (
The distribution of planidia observed in nectaries on leaves of Chilopsis may be due to one or several interacting factors. Both total number and qualitative condition of nectaries change along shoots of Chilopsis linearis (Fig. 2). The distribution of immature stages of Orasema simulatrix was not uniform along the branch, and although wasps preferred to oviposit next to nectaries, there was no correlation between eggs laid and total number of nectaries (Figs 2, 3). However, the most recent oviposition events, based on the presence of white, undeveloped eggs, were biased toward nectaries filled with fluid or at least wet (Fig. 5a). Older dry nectaries were generally least favored (Fig. 5a, b). Planidia, as indicators of old oviposition, were not present in the apical 1-10 leaves and the growing tip, except in the regrowth sample. There was a general progression of development of Orasema simulatrix along the branch, from undeveloped (light) to developed (dark) to planidia. Planidia were mostly found on older Category 3 and 4 leaves. In general, the more basal leaves had fewer nectaries, and on typical growth branches, these were mostly dry. Most nectaries were in Category 4 (31-40 leaves from growing tip), but this was the least favored area for oviposition. When nectary condition was more evenly distributed, as in the two regrowth samples, oviposition events were also more evenly distributed along branches. The placement of eggs appears to be biased toward the fresher leaves with the most fluid-filled nectaries, with planidia emerging near EFN that are most likely either fluid filled or wet. This would maximize encounters with foraging ant workers feeding at the nectaries. A direct association with the nectary, either on the rim of the nectary or within the fluid of the nectary, likely maximizes the chances for the planidia to be orally transferred along with the nectar/food by trophyllaxis to the ant larvae within the nest.
Overall, the evolution and behavior of Orasema simulatrix are tied to its ant host, with the wasp dependent on the host plant. This wasp is closely associated with a common desert plant that can harbor populations of an ant host such as Pheidole desertorum. Furthermore, both adult female wasps and planidia exhibit behaviors that increase the chances of encounter between planidia and their ant hosts.The use of extrafloral nectaries has not been documented for any ant parasitoid. A direct food-source interaction is known only for eucharitid parasitoids of camponotine ants, which lay their eggs into fruit attractive to the ants (
We thank Astrid Cruaud, Jason Mottern, John Hash and Elizabeth Murray for comments on earlier drafts of the manuscript. This research was supported by National Science Foundation grants DEB 0108245 and PEET DEB-0730616 to JMH.