(C) 2012 Christophe Barthélémy. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Field observations and rearing observations of five nests of Macromerella honesta (Smith) and four nests of Auplopus sp. were carried out in Hong Kong, China, in 2006, 2009, 2011 and 2012.
Nests of Macromerella honesta were composed of two rows of exposed ovoid mud cells, affixed to broad leafs while nests of Auplopus sp. were constructed as variable masses of mortar affixed to a linear substrate (thorns, wire-mesh, etc.). Cells of the nests of Auplopus sp. were more or less cylindrical and covered with a layer of hardened mud finished with a plant gum coating. Both species used a single dismembered spider, transported ventrally to the nesting site, for cell provisioning. Active guarding was observed at all nesting sites. Overlap of generation was also recorded with active nest cooperation (construction and prey provisioning) between individuals of different generations on the same nesting site. The two species were inferred to be at least bivoltine in Hong Kong.
Macromerella honesta, Auplopus, Pepsinae, cooperative behavior, defensive behavior, resin
Spider wasps (Hymenoptera: Pompilidae) are an interesting aculeate family, especially due to their diverse and varied natural histories with species ranging from ectoparasitoids of spiders to cleptoparasitic species, and from solitary to communal or parasocial. Species of the tribe Ageniellini (Pepsinae) are known to exhibit some of the most intricate nesting behaviors within the family, particularly with regards to nest construction. There is a general consensus on the steps that may have led from soil nesting to the building of more complex nests above ground, in some cases occupied by several individuals, in Ageniellini (
Observations by C.B on the nesting behavior of the two species of spider wasps were primarily carried out from April to November 2006 on a daily basis, weather permitting, mostly in this author’s former garden, Pak Sha O, Sai Kung, Hong Kong (22.15N-114.13E; UTM: 50Q KK 242 849, alt. 70 m asl.). Additional observations were carried out in September and October 2009, May and September 2010 and November 2011. Specimens of both spider wasp species are deposited in the collections of the Department of Biology Entomological at Utah State University, Logan, UT, USA (EMUS). Identification of Macromerella honesta was obtained using Yamane (1999) and J.P.P. identified Auplopus sp.
Five nests of Macromerella honesta were observed. Four nests, referenced Macromerella Nest 1, 3, 4 and 5 (Table 1), were located at Pak Sha O, while Macromerella Nest 2 was observed at Kau Lung Hang Shan; Fanling (UTM: 50Q KK 089 884, alt. 400m asl ). Also, four nests of Auplopus sp. were observed at Pak Sha O, and referenced Auplopus sp. 1 to 4 (Table 1).
Observation and collection periods for both species.
| Species | Nest Reference | Observation period | |
|---|---|---|---|
| Start | Finish | ||
| Macromerella honesta | Macromerella-Nest 1 | 23 Apr-2006 | 16 May 2006 |
| Macromerella-Nest 2 | 31-Aug-2006 | 31-Aug-2006 | |
| Macromerella-Nest 3 | 17-Jul-2006 | 17-Jul-2006 | |
| Macromerella-Nest 4. (Nest collected 17-Oct-2009 and reared) | 08-Oct-2009 | Nov-2009 | |
| Macromerella-Nest 5 | 12 June 2010 | 03 July 2010 | |
| Auplopus sp. | Auplopus sp-Nest 1 | 11-May 2006 | 14-Jun-2006 |
| Auplopus sp-Nest 2 | 02-Jun 2006 | 22-Jul-2006 | |
| Auplopus sp-Nest 3 | 28-Jun-2006 | 30-Jun-2006 | |
| Auplopus sp-Nest 4. (Nest collected 20-Sep-2009 and reared) | 20-Sep-2009 | Sep-2009 | |
The female has a body length of 12.2–16.7 mm (mean = 15.2 mm, n = 7) and a fore wing length of 11.7–13.4 mm (mean = 12.9 mm, n = 7). The head, mesosoma, coxae and metasoma are brilliant metallic bluish green as in Figs 11–12. The legs are brown with slight metallic bluish green reflections, while the antennae are black (Fig. 12). The wings are clear and have venation as in Fig. 13. The clypeus is convex in lateral view with the anterior margin rounded medially and sinuate laterally (Fig. 12). The body is covered in short white setae, which is most dense on the clypeus, face laterally and ventral to antennal scrobes, fore coxae, and mesosternum (Figs 11–12).
The male has a body length of 11.2–14.1 mm (mean = 12.7, n = 2) and a fore wing length of 10–11.8 mm (mean = 10.9, n = 2). Frons, mesosoma, dorsal side of mid and hind coxae, dorsal side of first terga and metasoma segments 2-7 are brilliant metallic bluish green as in Figs 14–15. The ventral side of coxae are light brown. The fore coxa are light brown with a brilliant metallic bluish green longitudinal band dorsally, while the ventral portion of the mid and hind coxae light brown. The front legs are light brown, the mid legs are brown with diffuse metallic bluish green reflection, and the hind legs are slightly darker. The face has two yellow bands along the eye margins terminating at the frons. The antennae are light brown with diffuse metallic bluish green reflection. The wings are clear and have venation as in Fig. 16. The clypeus is strongly convex in lateral view with the anterior margin flat medially and oblique laterally (Fig 14–15). The body is covered in short white setae which is most dense on clypeus, gena, ventral part of pronotum and lateroventral sides of fore coxae.
Results Description of nest and behavior of Macromerella honesta (Smith, 1855)Macromerella Nest 1 (Figs 1–3): This nest was initiated around 23 April 2006 and completed around 5 May 2006 at Pak Sha O; it was composed of nine cells in two rows. On 3 May 2006 one cell was found open and by 7 May a female wasp was seen rebuilding this cell using material from adjacent cells. The wasp remained on the cluster until the end of this observation. Normally the female wasp would rest on the dorsal part of the nest and inspect the surface at more or less regular intervals. The female wasp showed clear defensive behavior when the nest was approached closely by the observer. On 3 May the first individual (female) emerged and both females remained on the nesting site for another week. By 8 May adults had emerged from four cells, but only two individuals remained on the nesting site.
Macromerella Nest 1 with founding mother guarding the nesting site (7 May 2006).
Macromerella Nest 1 with overlap of generation on the nesting site (8 June 2006).
After emergence of the first offspring, the two individuals shared the nesting site and even provisioned one of the open cells with a new prey (Fig. 2). Neither oviposition nor feeding on the haemolymph of this prey by the adults was observed, although commonly reported (Wcislo 1987;
Macromerella Nest 1 with individual sheltering in an empty cell (5 June 2006).
Macromerella Nest 2 (Fig. 4): This nest was observed at Kau Lung Hang Shan on 31 August 2006 and photographed; it was affixed to a broad leaf of an unidentified bush/tree and was composed of 15–16 cells forming a large surface (no rows). Most cells were open but the nesting site was still occupied by one individual (male). This was most likely an early season nesting site, re-used by successive generations.
Macromerella Nest 2 showing irregular shaped structure possibly due to overlap of construction following occupation by two or more generations (31 August 2006).
Macromerella Nest 3: One wasp was seen initiating a cell on the underside of a broad leaf bush at Pak Sha O on 17 July 2006. No further observations were made.
Macromerella Nest 4 (Fig. 5): On 8 October 2009 one female wasp was seen guarding a nesting site at Pak Sha O composed of two mud cells and one unfinished cell located on the underside of a leaf of Lagerstroemia speciosa L, Queen Crape Myrtle; the nest was most likely initiated a short time earlier. The wasp was guarding the nest until 15 October 2009 and then disappeared. One female emerged on 2 November 2009 and one male on 5 November 2009.
Macromerella Nest 4. A small nest with a guarding female (8 October 2009).
Macromerella Nest 5: Inmid June 2010 two wasps were seen on a nest composed of six cells pasted on the upper side of a leaf of a Pomelo tree (Citrus grandis (L.) Osbeck) at Pak Sha O. By 23 June 2010 three wasps were seen although apparently no adults had yet emerged. The gender of the three adults was not ascertained. By 3 July 2010 wasps had emerged from five cells, the sixth cell containing a dead pupa in her cocoon.
Macromerella honesta supplied each cell with a single mature specimen of a large unidentified spider species. Prey transportation was not observed. Although the unused item of nest Macromerella Nest 1 was likely amputated, it is interesting to note that
This species builds multi-cellular nests on either side of broad leaves, bushes, or trees. The cells were composed of a plastic mud mixture (clay and sand grains) drying into an effective mortar. The cell walls were relatively thin, measuring approximately 1.5 mm at the apex of the dome and 2–4 mm at the base.
The construction materials were carried from the acquisition site to the nest in the mandibles of the wasps and most likely softened with regurgitated water. It was also noted on Macromerella Nest 1 that remoistened mortar from adjacent cells was used to re-model/re-construct other cells, the wasp having carried water in her crop to the nesting site. When applying the mortar to the cells, the wasp flexed its metasoma under the mesosoma and transferred a droplet of mortar to the tip of the metasoma; she then applied the mixture to the cell rim with a rapid back and forth motion of the metasoma.
Cells had an elliptical base and were broadly hemispherical; they were assembled in a cluster generally formed by two regular interlocking rows of 2–15 cells. The arrangement became unclear in re-used nesting sites, as additional cells were added and original ones remodeled (see Fig. 4). The external walls of the cells were not protected by an additional clay layer enclosing all the cells together as in other mud-nesting pepsine wasps (
The brood development time seemed to vary greatly in this species with larvae in Macromerella Nest 1 completing development in two weeks they took approximately one month in Macromerella Nest 4 .
The female actively defended the nest and C.B. directly experienced this behavior when a nesting site was disturbed. At the end of September 2009 while cutting down vines along a path C.B. was stung first on the hand by a defensive female and less than five minutes later on the head by the same individual. The sting was not overly painful, but soreness was felt the next day and for a few days afterwards.
Description of nest and behavior of Auplopus sp.Auplopus Nest 1, (Figs 6–8): This nest was first observed on 11 May 2006 at Pak Sha O. Field observations showed that nest building could be time consuming: the construction and provisioning of one cell would take a minimum of one day, sometimes more depending on the weather conditions. Therefore, with approximately eight cells completed at time of first observation we can infer that the nest was initiated around 1 May. It was affixed to a small branch of an unknown bush. At completion the nest had approximately 10 mud cells encased in an additional layer of mortar. The top of the structure was finished with a resinous plant gum or “lacquer” applied by the wasp.
Auplopus Nest 1 with unfinished cell at the bottom of the structure (11 May 2006).
Auplopus Nest 1 with original female provisioning a cell with a dismembered single prey (26 June 2006).
The nest was guarded by the initiating female at least until the first offspring emerged, which was assumed to be between the 3 and 7 June 2006, based on recorded observations. The emerging adults chewed a small hole (2–3 mm diameter) in the mortar layers and appeared head first. Although two exit holes were counted on the nest, only two individuals were present; one was small and presumably a male, while the other was larger and presumably a female, the other specimen(s) probably having left the site. Both individuals were present on the nest until 7 June 2006 and both were defensive of the nesting site when observations were being made. The female seemed to be the more aggressive of the two and held her ground while the male would fly off to a neighbouring position after repeated encounters. By 14 June all brood had emerged and the nesting site was abandoned.
Auplopus Nest 2, (Fig. 9): The nest was first observed on 2 June 2006 it had approximately four cells completed and the wasp was actively building new cells, therefore one can infer that it was initiated several days before (28 May). It was affixed to a young branch of a Citrus grandis tree. By 25 June there were 8–9 cells constructed in the same fashion as Nest 1, although here the resinous material was applied to the whole structure. Construction was hampered by a bout of inclement weather (seasonal rains from the 8 until 13, with a few heavy showers on 17 or so). On the 25th, the initiating female brought back to the nest a large spider with all appendages amputated save for the pedipalps (Fig. 7). By the 28th an adult wasp had emerged and was cooperating with the initiating female in nest building activities; both individuals were seen carrying construction materials to the nest; mortar for one individual and resin for the other. It could not be ascertained if each individual solely collected one type of construction material or not. This new individual also defended the nest and was presumed to be female, although the sex was not established with confidence.
Auplopus Nest 1 with original female building a ventral cell (11 May 2006).
Auplopus Nest 2 with resinous material applied to the entire structure, appearing as darker shades of yellow to red 30 June 2006).
The nest was collected on 30 June 2006 and placed in a plastic aquarium. From it emerged in chronological sequence: one female on 14 July, one female and two males on 18 July and one female on 22 July. One male never managed to exit completely and died. A total of six adults emerged from the nest of which four were female and two were male.
Auplopus Nest 3 (Fig. 10):The nest was first observed on 28 June 2006, it had approximately 10 cells completed and the wasp was actively initiating new cells, therefore one can infer that it was initiated at least 10 days before at a rate of approximately one cell a day. The structure was hanging from a large thorn of a citrus tree (Citrus grandis). The female was still adding cells and by 30 June 2006 observations were halted. This nest was the longest structure witnessed, reaching 12–15 cm and having approximately 12 cells. The construction was identical to Nest 1 and 2 and the resinous material covered the entire structure. By the time of observations later in the season, a few adults had emerged, evidenced by four exit holes, although the usage of the same exit hole by several specimens cannot be ruled out. No additional adults had emerged by the spring of the following year.
Auplopus Nest 3, it was the largest nest encountered (30 June 2006).
Auplopus Nest 4: The nest was discovered on 20 September 2009 affixed to wire mesh (1.5 × 1.5cm) inside an open garden shed. The nest was broken when detached revealing three cells out of a total of five. One of the open cells contained a live pupa but the other two were empty. The nest was reared inside a Ziploc bag and one male emerged on 21 September 2009 and one female on 25 September 2009. Plant gum was apparently not applied to the outer surface.
Female Auplopus sp., head, frontal view.
Female Auplopus sp., lateral view.
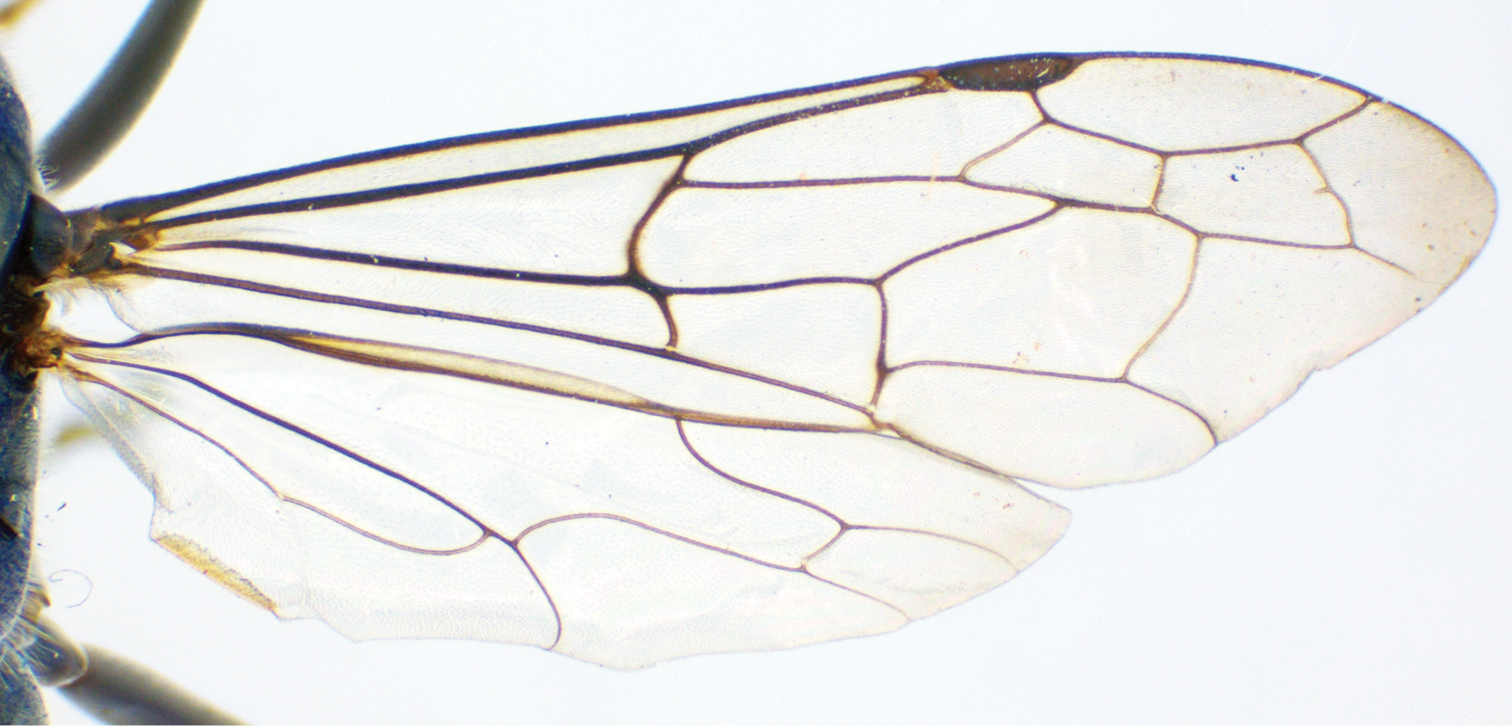
Female Auplopus sp., fore wing.
Male Auplopus sp., head, frontal view.
Male Auplopus sp., lateral view.
Male Auplopus sp., fore wing.
May 2010: C.B. observed Auplopus sp. transporting a spider in Pak Sha O, The spider was grasped by its spinnerets and carried ventrally with the ventral side of its abdomen up. The wasp had not amputated the prey, which was of small size. The wasp was not observed to fly with the spider.
September 2010 and November 2011: Observations by C.B in Pak Sha O revealed that wasps would gather construction material from termite nests, and would rehydrate the dirt to form a ball of material, suggesting that water or nectar was acquired beforehand. Three nests affixed to wire mesh in a garden shed did not have any plant gum applied externally.
May 2012: C.B. found a nest at Pak Sha O affixed to wire mesh in a garden shed. The initiating wasp was present building a new cell. The nest was covered with plant gum.
The Auplopus sp., as with other Pompilids, supplied each cell with a single mature prey specimen. The wasp often amputated the legs of the spider; the wasp carried the spider ventrally, abdomen up, grasped by the spinnerets. The female deposited the paralyzed prey in a pre-constructed cell, oviposited on it and sealed the cell.
This species constructed a cluster of 5–12 near cylindrical cells hanging from a linear (curvy-linear) substrate (twig, thorns, petioles, etc.). The cells were further aggregated in an additional layer of mortar enclosing the whole structure. The whole construction was of irregular shape, although elongated. The construction material was obtained from dry sources rehydrated with regurgitated water.
In addition Auplopus sp. sometimes applied a resinous material to the hard external envelope of the nest. This material was applied first to the dorsal surface of the nest and formed a varnish-like, brownish, translucent layer. The wasp carried the resinous material c in her mandibles and applied it as she would mortar. This substance was sticky at first, but hardened over a period of 10–15 days. The mode of transportation and application of the construction materials was identical to that of Macromerella honesta (Fig. 8).
Auplopus sp.’s development time (oviposition to emergence of adult) was between one month and 1.5 months, as inferred from the small set of observation (Auplopus Nest 1 and 2). The species was active at building nests from at least May to November.
DiscussionParticular behavioral characteristics of Macromerella honesta and Auplopus sp. are worthy of mention. The nesting hierarchy of both species strictly follows
Many aerial nests of members of Ageniellini described in the literature, such as the Auplopus sp. described here, are generally an aggregation of several cells covered by an additional mortar layer (Wcislo 1987;
Macromerella honesta and Auplopus sp. constructed brood cells by transferring material from the mandibles to the tip of the metasoma, which was used as trowel, a behavioral trait seen in other species of pompilid wasps, either to plaster mud or ram soil at the nesting site (
Using an additional finishing layer of resinous plant gum, as seen here in the Auplopus sp., has rarely been described before in the tribe, although Auplopus nyemitawa (Rowher 1919) is known to use both a resinous gum and lichens applied as a finishing layer on nest structures (
The construction of the nest in both species generally was carried out solely by the initiating female, although nesting sites might be reused by overlapping generations (Macromerella Nest 1 and Auplopus Nest 2). Data on the communal construction activity noted for Auplopus sp. is insufficient to ascertain if this behavior was productive of a viable and durable new nesting site or if it was just incidental.
Active nest construction for both species generally ends towards September/October locally, although C.B saw one individual of Auplopus sp. actively building a nest in November 2011, a year that was particularly mild in Hong Kong.
The overlap of generations on the nesting siteGenerational overlap is rarely described in wasps other than in Vespidae and Sphecidae. However, a number of species in the Pompilidae are known for having a communal nesting biology, such as communal defense or nest building (Kimsey 1980;
For the Auplopus sp., however, the generational overlap seemed to involve more elaborate behaviors than those of Macromerella honesta. Besides communal defense, the Auplopus sp. also exhibited communal nest building, with two individuals performing separate tasks: one building cells, and the other varnishing the structure, although roles may well have be exchanged in the course of construction.
Prey transportationBoth species carried their prey ventrally, with the spider’s abdomen up. Amputation, however, seemed to vary in Auplopus sp. and may have depended on the size of the prey. Whether or not Macromerella honesta amputates the legs of its host was not ascertained. Carrying prey ventrally is recognized as being a derived condition evolving from the more primitive condition of dragging the prey by its appendages to the nesting site, which is seen in many Ageniellini (
While the pedipalps of both species were intact, Auplopus sp. has been observed holding the spider’s spinnerets to place the prey in an empty cell (Fig. 7).
VoltinismThe two weeks to one month development period of Macromerella honesta, combined with continuous observations of the species during the summer months (May to October) in Hong Kong and the presence of an active nest in early October suggests that this species was at least bivoltine if not multivoltine in Hong Kong. A single observation of a female actively searching leaves of a camel foot tree (Bauhinia sp.) in a garden in Pak Sha O, Hong Kong on 17 December 2006, long after the emergence of the last generation, may suggests that some females overwinter, although no such observation has been directly recorded with the nesting individuals. The development stage in which this species over-winters has not been established. However, it most likely does so as a diapausing pre-pupal larva.
Similarly, the one month development time for Auplopus sp. combined with observation of nesting individuals in November, suggest that this species was at least bivoltine if not multivoltine in Hong Kong. The development stage in which this species over-winters has not been established, but likely does so as a diapausing prepupa.
ConclusionNest location, nest construction, prey transportation and prey provisioning of Macromerella honesta and Auplopus sp. are highly complex when compared to other members of the family.
While the two species described here adhere quite tightly to the widely accepted nesting hierarchy of agenielline wasps, it is interesting to note that many species are known to exhibit a relative plasticity of nesting behavior intraspecifically (
The location and the construction of the cell aggregates and the transportation of the prey items are traits that place Macromerella honesta and Auplopus sp. among the more complex of Pompilidae. Indeed some authors view the evolution of the nesting biology from ground cavity dwellings to aerial abodes as a precursor of presocial or parasocial behavior (Evans 1996, Shimizu 1998). It would be tempting to categorize these two species as presocial, but the data gathered here is inconclusive and rather suggest probable communal nesting.
We are grateful to one anonymous reviewer and the editor JHR who suggested useful modifications to the original manuscript. This work was also supported by the National Science Foundation award DEB-0743763 to J.P.P. This research was also supported by the Utah Agricultural Experiment Station, Utah State University, Logan, Utah 84322-4810. Approved as journal paper no. 8411.