(C) 2012 David R. Smith. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Five species of Trigonalidae, hyperparasitoids of Ichneumonidae (Hymenoptera) and Tachinidae (Diptera) that parasitize caterpillars (Lepidoptera), have been reared during the ongoing caterpillar inventory of Area de Conservación Guanacaste (ACG), Guanacaste Province, northwestern Costa Rica: Lycogaster apicipennis (Cameron), Taeniogonalos woodorumSmith, sp. n., Taeniogonalos fasciatipennis (Cameron), Trigonalys championi Cameron, and Trigonalys maculifrons Sharp. Morphological and DNA barcoding data support species separation of these generalist hyperparasitoids. Taeniogonalos gundlachii (Cresson) is not a widespread, color-variable species as previously treated and is probably confined to eastern North America. The species previously considered as Taeniogonalos gundlachii in Costa Rica is regarded as Taeniogonalos fasciatipennis, a species found only in ACG dry forest. Taeniogonalos woodorum is a similar species but found only in the ACG rain forest. Habitat and host records are given for these five species of trigonalids.
Central America, hyperparasitoid host specificity, Lepidoptera, Diptera, DNA barcoding, tropical trophic web
Trigonalid wasps are usually hyperparasitoids, parasitizing Ichneumonidae (Hymenoptera) and Tachinidae (Diptera) larvae that parasitize Lepidoptera caterpillars, or they are parasitoids of the larvae of social or possibly solitary wasps (
Ten species in six genera of Trigonalidae have been recorded from Costa Rica (
The major identification problem among the reared species is that which appears morphologically to be the color-variable and widespread species Taeniogonalos gundlachii (Cresson). This has been considered to be a single species, known from southeastern Canada to Cuba and Central America, following the synonymy by
Here, we present morphological and DNA analysis evidence for the distinctiveness of the five reared species, one of which is new, and offer rearing records and hosts.
Materials and methodsImages were obtained using an EntoVision Imaging Suite that included a firewire JVC KY-75 3CCD digital camera mounted to a Leica M16 zoom lens via a Leica z-step microscope stand. Multiple focal planes were merged using Cartograph 5.6.0 (Microvision Instruments, France) software.
Acronyms used are: The Natural History Museum, London, UK (BMNH); Canadian National Collection of Insects, Ottawa (CNC); Instituto Nacional de Biodiversidad, Santo Domingo de Heredia, Costa Rica (INBio), National Museum of Natural History, Smithsonian Institution, Washington, D.C., USA (USNM). Other specimens examined in this study were kindly loaned from the Florida State Collection of Arthropods, Gainesville, FL, USA, and the collection of N. M. Schiff, Stoneville, MS, USA.
In the Costa Rican ACG inventory, each reared adult receives one voucher code, and often a second. The invariant voucher code of the form “99-SRNP-4597” (year-Santa Rosa National Park-unique rearing number) is unique for the event of finding and rearing the caterpillar, irrespective of what it produces. The entire rearing record may be obtained by searching with this code at http://janzen.bio.upenn.edu/caterpillars/database.lasso. If a hyperparasitoid wasp is DNA barcoded or otherwise treated as an individual (a “daughter specimen” of the SRNP rearing event) it is assigned a second unique voucher code of the form DHJPAR0034526 which applies only to that individual. This voucher code may be used to obtain the entire caterpillar (and wasp) rearing record from the same website and as described in
When rearing wild-caught caterpillars in the ACG inventory, wasp cocoons and tachinid fly puparia are routinely obtained and held for adult wasp and fly eclosion. There is no way to know if the wild-caught caterpillar has primary parasitoids in it, or if they in turn are hyperparasitized. Usually, primary parasitoids that are uninfected and emerge from a single caterpillar (or pupate in a caterpillar or pupal mummy) eclose during a 1–3 day period after 1–3 weeks (with a few exceptional univoltine species of primary parasitoids remaining dormant for 10–12 months). If the pupae inside these wasp cocoons or fly puparia contain a hyperparasitoid (Trigonalidae, Perilampidae, Eulophidae, or Ichneumonidae), this insect tends to eclose a few days to weeks after the eclosion of the primary parasitoid. This delay necessitates retaining and continuing to observe “dead” primary parasitoid wasp cocoons and tachinid puparia. This is especially the case if there are, for example, five tachinid puparia from one caterpillar and three of them produce tachinid flies; the remaining two are usually dead “of disease” but on rare occasions produce a trigonalid or other hyperparasitoids.
Eclosed trigonalids were killed by freezing, as are other ACG inventory insects, pinned, labeled with the above-described yy-SRNP-xxxxx label, stored frozen, and then oven-dried (40–55°C). In the University of Pennsylvania clearing center, one leg was removed and couriered to the Biodiversity Institute of Ontario (BIO) at the University of Guelph for DNA extraction, barcode amplification, and accessioning in the Barcode of Life Data System (www.boldsystems.org). At this time the leg and the corresponding specimen also received the second above-mentioned DHJPARxxxxxx voucher code, which was used in all DNA barcode analyses of ACG parasites and parasitoids. If the wasp is very small, it may be collected into 95% ethanol for refrigerated storage instead of pinned and oven-dried. This treatment of hyperparasitoids by the inventory does not differ from that of primary parasitoids, except that more patience is required in waiting longer for hyperparasitoids to eclose.
Field identification (and later corroboration) of the host caterpillar is done by the inventory parataxonomists, later by DHJ and WH as inventory coordinators, using photographs, host remains, food plants, and microgeographic characters of caterpillars. Laboratory identification of the primary parasitoid remains was done by DHJ using intense familiarity and photographs and reference specimens with the parasitoids and their body parts (cocoons, pupae, puparia) as well as ecological information such as the identity of siblings that were not hyperparasitized. Nearly all reared parasitoids were also DNA barcoded to tease out cryptic species complexes (e.g.,
DNA extracts for 201 trigonalid specimens were prepared from single legs using a glass fibre protocol (
Five species of trigonalids are recognized from the ACG rearings. DNA barcoding suggests the existence of four entities (Figs 1, 2) of Taeniogonalos within the ACG. Taeniogonalos woodorum, occurring only in ACG rain forest, is clearly distinct genetically and morphologically from the others and is here described as new. We find Taeniogonalos fasciatipennis from the lowland dry forest to be distinct from the eastern North American Taeniogonalos gundlachii. Although there is genetic evidence for separation of Taeniogonalos fasciatipennis into two species, we treat them here as one, but suggest that further research will reveal that this single name covers two species, here called Taeniogonalos fasciatipennis DHJ001 from the driest parts of lowland dry forest and Taeniogonalos fasciatipennis DHJ002 from the upper, cooler, and more moist portion of the lowland dry forest (see discussion under Taeniogonalos fasciatipennis).
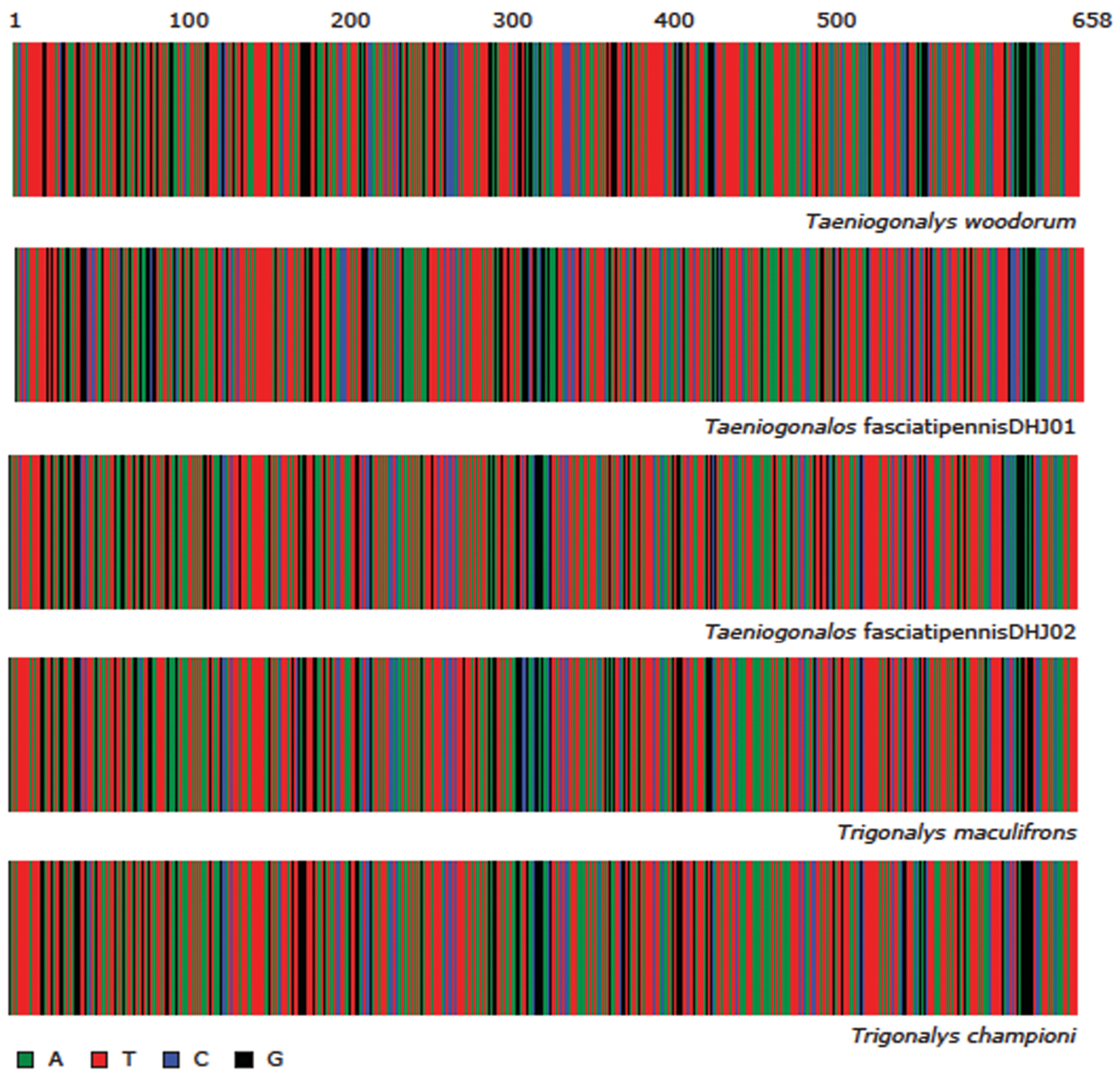
Color representation of the full length (658 base pairs (bp)) DNA barcodes for each of the 5 ACG trigonalid species. Intra-specific variation in the barcode region is represented by vertical bands in the color bar at that position.
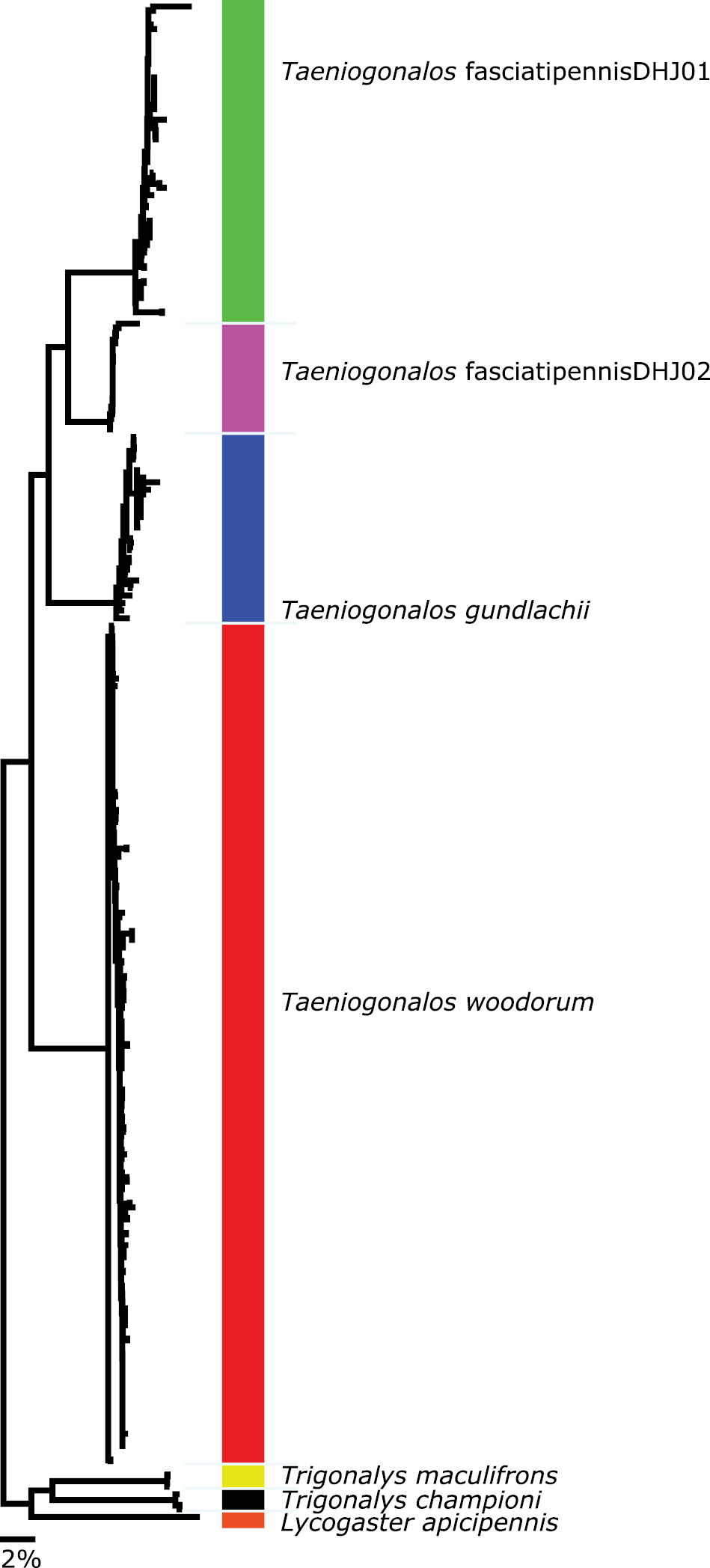
A neighbor-joining tree (NJ) built using Kimura 2 Parameter distance and including 201 sequenced trigonalid specimens from the ACG and North America that have COI sequence greater than 200 bp. Note the divergence between the ACG Taeniogonalos and the North American specimens – and within the dry forest Taeniogonalos fasciatipennisDHJ01 and Taeniogonalos fasciatipennisDHJ02 – all are clearly differentiated by mitochondrial DNA.
The very low percent yield (0.05%) of trigonalid wasps from wild-caught ACG caterpillars is partly generated by the natural history observation that trigonalids are usually not hyperparasitoids of primary parasitoids of leaf-rolling and leaf-tier caterpillars (Pyralidae, Thyrididae, Crambidae, Elachistidae, Tortricidae, etc.), which make up at least 20% of the caterpillars sampled and largely live their lives inside of leaf rolls. In other words, if just the more exposed caterpillars of the inventory are taken into account, the percent yield of trigonalids increases to (0.07%).
The inventory has to date logged about 47, 000 parasitized caterpillars (9.6% parasitization frequency), and 0.59% of these yielded one or more trigonalid wasps. However, 15, 000 of these were attacked by Eulophidae and Microgastrinae Braconidae) generally too small to support the much larger trigonalid larva. For the remaining primary parasitoids (Diptera and Hymenoptera), the hyperparasitization frequency was 0.86%.
The Trigonalidae rearing experience to date shows almost no examples of sharing primary parasitoids hosts with other hyperparasitoids (Perilampidae, Mesochorus in the Ichneumonidae, Eulophidae, and Chalcididae). That is to say, when a clutch of tachinid puparia or microgastrine braconid cocoons produce a trigonalid wasp(s), that is the only hyperparasitoid that emerges from this clutch of primary parasitoids except in one case of sharing with a perilampid (83-SRNP-1127.2) and one case of sharing with a chalcidid (83-SRNP-1432).
Trigonalidae identification by morphology alone is made complicated by as much as 20 times variation in body weight of conspecifics, owing to the body size being determined in great part by the body size of the primary parasitoid host larva. DNA barcoding, however, of the ACG reared trigonalids has demonstrated unambiguously that specimens of this huge size range, from the same place in ACG, are the same species.
In the future, it may be possible to determine the primary parasitoid host of a wild-caught adult trigonalid through DNA barcoding of its gut content (e.g.,
Keys to Central American and Neotropical genera of Trigonalidae were provided by
*Lycogaster apicipennis (Cameron, 1897).
Nomadina smithii Westwood, 1868. Described from “Amaz.” Recorded from Costa Rica by
Seminota depressa (DeGeer, 1773). Recorded from Brazil, Bolivia, Peru, Costa Rica by
Seminota laeviceps (Cresson, 1879). Described from Mexico. Recorded from Costa Rica, Panama by
*Taeniogonalos fasciatipennis (Cameron, 1897).
*Taeniogonalos woodorum (described below).
Taeniogonalos ornata (Smith, 1861). Recorded from Mexico and Costa Rica by
*Trigonalys championi Cameron, 1897.
*Trigonalys maculifrons Sharp, 1895.
Xanthogonalos robertibuyssoni Schulz, 1907. Described from Mexico. Recorded from Costa Rica by
http://species-id.net/wiki/Lycogaster_apicipennis
Figure 3This is the only species of Lycogaster known from Central America. It is distinguished by its spindle-shaped antennae, without tyloids, and with the basal 3 flagellomeres reddish brown (Fig. 3). The head and thorax are mostly black with only the tegula and spot on upper mesopleuron yellow, and the head and body are covered with golden-yellow hairs. The wings are yellowish, darker anteriorly and at apices, with the veins yellowish and stigma black.
Lycogaster apicipennis is between 16–18% different (K2P distance model) from other Trigonalidae in the ACG in the CO1 mtDNA barcode region.
Lycogaster apicipennis female, lateral view.
Costa Rica, Mexico (
10, 7 of which are barcoded. Deposited in USNM, INBio.
The ACG caterpillar inventory has reared Lycogaster apicipennis 10 times (between 1990 and 2008), and always in lowland dry forest. Six rearings have been from Enicospilus flavostigmusDHJ02 (Ichneumonidae) parasitizing Boriza crossaea Druce (Notodontidae), once from Enicospilus flavostigma Hooker parasitizing Dicentria rusticaDHJ05 (Notodontidae), two from Cubus validusDHJ03 (Ichneumonidae) parasitizing Omiodes cuniculalis Guenée (a large leaf-rolling Crambidae), respectively, and once from Bassus brooksi Sharkey (a large solitary Agathidinae Braconidae parasitizing Epargyreus in the Hesperiidae). If these primary parasitoid genera are viewed as the possible host universe, 2, 377 caterpillars attacked by them yielded 10 Lycogaster apicipennis (0.42% frequency). Alternatively, if we use the genera of the host caterpillars (Boriza, Dicentria, Omiodes, Epargyreus) in the inventory as the available universe, 17, 007 reared wild caught caterpillars yielded these ten Lycogaster apicipennis (0.059% frequency). This is a low density hyperparasitoid. The first six rearings (1990–1995) were all from Enicospilus flavostigmusDHJ02 parasitizing Boriza crossaea in ACG, and it would have been reasonable to label this wasp as a specialist to this host combination, but subsequent rearings makes it evident that it is at best a specialist on relatively large primary parasitoid wasps (and there is no suggestion that it is a hyperparasitoid of tachinid fly larvae, despite their being commonplace primary parasitoids of Boriza crossaea).
urn:lsid:zoobank.org:act:32992182-0692-42C7-8CF1-9166C53733A6
http://species-id.net/wiki/Taeniogonalos_woodorum
Figures 4–7Posterior postocellar area with two yellow oblique stripes; mandible mostly yellow, teeth reddish brown; gena mostly yellow, yellow extending dorsally and onto occiput; mesoscutellum with anterior third yellow; second metasomal tergum with narrow yellow band continuous along lateral and posterior margins. Female armature absent. Male with medial flattened area on sternum 2; genitalia with parameres about half as long as gonostipes.
Female. Length, 5.0–12.0 mm (holotype 8.0 mm). Antenna black. Head black, with following yellow (Figs 4, 5): Interantennal area, supraclypeal area, clypeus, labrum, mandible except reddish-brown apex, mouthparts, two oblique stripes at posterior or postocellar area, broad stripe on gena behind eyes extending dorsally onto occiput. Mesosoma black with following yellow markings (Figs 4, 5): anterolateral spot on mesoprescutum, axillae, anterior third of mesoscutellum, metascutellum, stripe on upper pronotum and lower pronotum, broad oblique stripe on mesopleuron, broad oblique stripe on metapleuron, large lateral spots on propodeum. Legs black with inner surfaces of coxae, trochanters, extreme bases of femora, and outer surfaces of tibiae and tarsi yellow. Metasoma black with following yellow markings (Figs 4–6): broad stripe on posterior margin of tergum 1, narrow continuous stripe on posterior and lateral margins of tergum 2; narrow stripe on posterolateral margins of terga 3 and 4, small spot on posterior lateral margin of sternum 2. Wings hyaline, black anterioapically, mostly in radial cell; veins and stigma black. Head and body covered with fine, short, white hairs. Head: Covered with short, white hairs. Shiny, evenly punctate, punctures mostly separated by rounded ridges, interspaces less than puncture diameters; punctures on gena less dense, farther apart than those on vertex and frons, and with flat shiny interspaces. Antenna with 24 flagellomeres, length about twice head width. Clypeus with median length about .3× width. Inner margins of eyes subparallel, lower interocular distance 0.7× eye length; malar space about 0.15× eye length. Distance between eye and margin of lateral ocellus about 3.0× distance between inner margins of hind ocelli. Area between torruli slightly concave (Fig. 5). Antennal carinae low, sharp. Occipital carina complete. Mesosoma: Covered with short, white hairs. Shiny, evenly punctate with punctures similar to those on vertex and frons, most separated by rounded ridges, with interspaces less than puncture diameters; punctures on propleuron farther apart than those on mesonotum and separated by broader, flat interspaces; dorsoposterior section of mesepisternum, posterior downturned margin of mesoscutellum, and metapleuron, except lower margin, almost impunctate; punctures on propodeum small, denser than those on rest of mesosoma. Prescutum elevated above lateral lobes. Notaulus deep, with large punctures posteriorly; transverse row of large punctures anterior to mesoscutellum. Propodeal foramen shallowly concave at center. Hind coxa about as long as wide, with longitudinal carina on outer surface; hind basitarsomere subequal to length of remaining tarsomeres combined. Metasoma: Covered with fine, white hairs. Shiny, rather evenly punctate, punctures separated by rounded ridges mostly less than puncture diameters. Tergum 1 depressed at center. Armature absent from sternum 2 (Figs 4, 6). Sheath directed downward, rounded at apex in lateral view.
Taeniogonalos woodorum. 4 Lateral view, female 5 Dorsal view, female 6 Metasoma, lateral view, female 7 Male genitalia.
Taeniogonalos fasciatipennis, female. 8 Taeniogonalos fasciatipennisDHJ01, lateral view 9 Taeniogonalos fasciatipennisDHH01, dorsal view 10 Taeniogonalos fasciatipennisDHJ02, lateral view.
Taeniogonalos fasciatipennis DHJ01. 11 Metasoma, lateral view, female 12 Female armature, sternum 2, ventral view 13 Male paramere, lateral view 14 Male genitalia.
Male. Length, 4.0–7.5 mm. Color and structure similar to female. Tyloids present, long and narrow, middle tyloids longer than half length of flagellomeres. Male genitalia with parameres about half-length of gonostipes (Fig. 7); sternum 2 with medial flattened area on apical half.
Taeniogonalos gundlachii. 15 Lateral view, female 16 Dorsal view, female 17 Metasoma, lateral view, female 18 Female armature, sternum 2, ventral view 19 Male paramere, lateral view 20 Male genitalia.
Holotype female, “Voucher: D. H. Janzen & W. Hallwachs, DB: http://Janzen.sas.upenn.edu, Area de Conservation Guanacaste, Costa Rica, ” “10-SRNP-22162, ” “DHJPAR0041177.” (USNM). Paratypes: Same data as for holotype except caterpillar rearing code (yy-SRNP-xxxx) and parasitoid individual code (DHJPARxxxxxxx); one specimen for each caterpillar collection and parasitoid rearing code. 98-SRNP-6785, DHJPAR0010613 (♂); 98-SRNP-7262, DHJPAR0016904 (♂); 98-SRNP-7361, DHJPAR0016911 (♀); 98-SRNP-15545, DHJPAR0016916 (♀); 98-SRNP-15545, DHJPAR0016899 (♀); 98-SRNP-15545, DHJPAR0016888 (♂); 99-SRNP-5503, DHJPAR0016895 (♀); 99-SRNP-5508, DHJPAR0016897 (♂); 99-SRNP-12098, DHJPAR0016891 (♀); 99-SRNP-12098, DHJPAR0016892 (♂); 99-SRNP-12098, DHJPAR0016896 (♀); 99-SRNP-12761, DHJPAR0010612 (♀); 99-SRNP-12852, DHJPAR0010604 (♂); 99-SRNP-13819, DHJPAR0010611 (♂); 99-SRNP-13823, DHJPAR0016909 (♀); 01-SRNP-3507, DHJPAR0010598 (♀); 01-SRNP-3507, DHJPAR0010599 (♀); 01-SRNP-5325, DHJPAR0010597 (♂); 01-SRNP-5932, DHJPAR0010605 (♀); 01-SRNP-9359, DHJPAR0010607 (♀); 01-SRNP-25186, DHJPAR0010600 (♂); 02-SRNP-7679, DHJPAR0010596 (♀); 02-SRNP-7978, DHJPAR0010595 (♀); 03-SRNP-6738, DHJPAR0010588 (♂); 03-SRNP-10070, DHJPAR0010585 (♀); 03-SRNP-11855, DHJPAR0010591 (♂); 03-SRNP-11855, DHJPAR0010592 (♂); 03-SRNP-11855, DHJPAR0010593 (♂); 03-SRNP-11855, DHJPAR0010594 (♂); 03-SRNP-20157, DHJPAR0010590 (♀); 03-SRNP-20236, DHJPAR0028047 (♀); 03-SRNP-21817, DHJPAR0010586 (♀); 04-SRNP-30072 [no barcode] (♀); 04-SRNP-41595, DHJPAR0010574 (♂); 04-SRNP-55214, DHJPAR0010571 (♀); 04-SRNP-55214.1, DJHPAR0010572 (♀); 04-SRNP-55215, DHJPAR0010573 (♀); 04-SRNP-56432, DJHPAR0010581 (♀); 04-SRNP-56458, DHJPAR0010582 (♀); 05-SRNP-4939, DHJPAR0010559 (♂); 05-SRNP-7881, DHJPAR0010551 (♂); 05-SRNP-33818, DHJPAR0010569 (♂); 05-SRNP-34358, DHJPAR0010562 (♂); 05-SRNP-42584, DHJPAR0010563 (♂); 05-SRNP-42827, DHJPAR0010570 (♂); 05-SRNP-70122, DHJPAR0010560 (♂); 05-SRNP-70325, DHJPAR0010561 (♀); 06-SRNP-6781, DHJPAR0016876 (♀); 06-SRNP-6781, DHJPAR0016877 (♀); 06-SRNP-6781, DHJPAR0016878 (♂); 06-SRNP-6781, DHJPAR0016884 (♀); 06-SRNP-30294, DHJPPAR0010443 (♂); 06-SRNP-30295, DHJPAR0010554 (♂); 06-SRNP-33412, DHJPAR0016873 (♀); 06-SRNP-34200, DHJPAR0016875 (♀); 06-SRNP-34577, DHJPAR0016886 (♀); 06-SRNP-34579, DHJPAR0016887 (♂); 06-SRNP-42284, DHJPAR0010555 (♀); 06-SRNP-42284, DHJPAR0010556 (♂); 06-SRNP-42284, DHJPAR0010557 (♂); 06-SRNP-42814, DHJPAR0016882 (♀); 06-SRNP-42819, DHJPAR0016883 (♀); 06-SRNP-43560, DHJPAR0016874 (♀); 06-SRNP-65304, DHJPAR0016885 (♂); 08-SRNP-2414, DHJPAR0027762 (♂); 08-SRNP-2414, DHJPAR0027763 (♂); 08-SRNP-6017, DHJPAR0030373 (♂); 08-SRNP-32269, DHJPAR0030377 (♀); 08-SRNP-41835 [no barcode] (♀); 08-SRNP-42172, DHJPAR0030374 (♀); 08-SRNP-42172, DHJPAR0030375 (♀); 08-SRNP-42172, DHJPAR0030376 (♀); 09-SRNP-2888, DHJPAR0036406 (♀); 09-SRNP-5008, DHJPAR0036682 (♀); 09-SRNP-32681, DHJPAR0038544 (♀); 09-SRNP-32752, DHJPAR0038545 (♀); 09-SRNP-32752, DHJPAR0038546 (♀); 09-SRNP-33385, DHJPAR0038543 (♂); 09-SRNP-69541, DHJPAR0036404 (♂); 09-SRNP-70610, DHJPAR0036405 (♀); 09-SRNP-73449, DHJPAR0037846 (♂); 09-SRNP-80526, DHJPAR0037847 (♂); 10-SRNP-1030, DHJPAR0039355 (♀); 10-SRNP-4609, DHJPAR0041181 (♀); 10-SRNP-22641, DHJPAR0041178 (♂); 10-SRNP-32041, DHJPAR0041179 (♂); 10-SRNP-42215, DHJPAR0041180 (♀); 10-SRNP-73124, DHJPAR0041176 (♀); 10-SRNP-73289, DHJPAR0041174 (♀); 10-SRNP-80666, DHJPAR0041175 (♂); 11-SRNP-2784, DHJPAR0045823 (♀); 11-SRNP-2784, DHJPAR0045824 (♂); 11-SRNP-2859, DHJPAR0044983 (♀); 11-SRNP-2911, DHJPAR0045822 (♂); 11-SRNP-71666, DHJPAR0045825 (♂); 11-SRNP-80954, DHJPAR0044984 (♀). Deposited in INBio, USNM, CNC, BMNH.
03-SRNP-38118, DHJPAR0010587 (metasoma missing); 06-SRNP-6781, DHJPAR0016872 (metasoma missing); 09-SRNP-72860, DHJPAR0040090 (broken).
100; 98 submitted for DNA barcoding, 89 of which yielded complete DNA barcodes publically available from BOLD.
Taeniogonalos woodorum is named in honor of Monty and Grace Wood of Ottawa, Canada in recognition of their three decades of intense and enthusiastic taxonomic and spiritual participation in the tachinid fly inventory of Area de Conservación Guanacaste and in INBio’s inventory of the flies of Costa Rica.
The DNA barcodes of the 89 Taeniogonalos woodorum specimens longer than 400 bp have less than 1% intraspecific divergence (0.702% avg, max 2.523%, min, 0%). They are 9% divergent from the DNA barcodes of Taeniogonalos fasciatipennis DHJ01 and Taeniogonalos fasciatipennis DHJ02.
The mostly black color with yellow maculation, as described and illustrated, and lack of female armature on metasomal sternum 2 help distinguish this species from most other New World species of Taeniogonalos. The females of Taeniogonalos fasciatipennis, Taeniogonalos gundlachii, Taeniogonalos lugubris (Westwood), and Taeniogonalos ornata (Smith) have distinct armature on sternum 2, and the latter three are mostly yellow or reddish-brown with black maculation. The females of Taeniogonalos enderleini (De Santis) and Taeniogonalos jucunda (Westwood) from South America lack female armature. Taeniogonalos enderleini occurs in southeastern Brazil, is mostly black with some yellow maculation, but the posterior lower part of the mesopleuron and the metapleuron are reddish brown and the postocellar area lacks yellow marks. Taeniogonalos jucunda (Westwood) was described from “Amaz.”, and the color was described as mostly reddish brown, head yellow, and the scutellum black, all of which differ from the color of Taeniogonalos woodorum.
Taeniogonalos woodorum is the most frequently reared of the ACG Trigonalidae, known only from ACG rain forest, and superficially resembles Taeniogonalos fasciatipennis and Taeniogonalos gundlachii (see below). It is the only species of trigonalid that has been reared from the sample of more than 220, 000 wild-caught ACG rain forest caterpillars. This microgeographic and ecosystem separation from the parapatric and adjacent ACG dry forest of Taeniogonalos fasciatipennis (see below) allows first-pass species-level identification of Taeniogonalos woodorum even if key morphological traits are invisible and DNA barcodes have not been obtained from the specimen, such as when the reared wasp escapes or the abdomen is broken off and lost or consumed in analysis. This method of ecology-based identification cannot, however, be used for specimens from the narrow ecotone between ACG dry forest and rain forest, where both species of Taeniogonalos have been reared from caterpillars found in the same hectare. The presence of Taeniogonalos woodorum was first noticed in 2006 by its strikingly different (15%) DNA barcode from that of Taeniogonalos fasciatipennis (also called Taeniogonalos gundlachii at that time). Adult Taeniogonalos woodorum, as is the case with the other ACG Taeniogonalos, is a Batesian and Mullerian mimic of Polybia wasps (Vespidae; abundant in ACG) in body size, color pattern, and flight/walking behavior.
Taeniogonalos woodorum has been reared 97 times from 14 caterpillar families (Arctiidae, Crambidae, Elachistidae, Geometridae, Hesperiidae, Lasiocampidae, Noctuidae, Notodontidae, Nymphalidae, Pyralidae, Saturniidae, Sphingidae, Thyrididae, Uraniidae), parasitized by Braconidae (Bassus, Dolichogenidea, Glyptapanteles, Meteorus, Stantonia, Zelomorpha), Ichneumonidae (Agrypon, Charops, Dusona, Eiphosoma, Hyposoter, Leurus, Microcharops, Xiphosomella, Zaglyptomorpha) and Tachinidae (at least Anoxynops, Agryrochaetona, Argyrophylax, Belvosia, Calolydella, Campylochaeta, Chrysotachina, Drino, Eucelatoria, Eujuriniodes, Eumea, Genea, Houghia, Hyphatrophaga, Lespesia, Patelloa, Phytomyptera, Winthemia). The host caterpillars of these primary parasitoids may all be characterized as exposed leaf feeders (even these particular species of leaf rollers and tiers, Crambidae, Elachistidae, Pyralidae, Thyrididae, venture out of their leaf-silk nests to feed on fully exposed leaf blades), and no one species dominates this diverse list. While it is evident that Taeniogonalos woodorum can develop in a very wide variety of host caterpillars and primary parasitoids, experience with other apparent “generalists” in the ACG inventory warns that when much larger sample sizes have accumulated, it may become evident that certain taxa and ecologies are either being avoided by ovipositing wasps or the eggs/larvae do not survive their tour in the host or primary parasitoid.
It remains a mystery as to why this hyperparasitoid remains microgeographically restricted to ACG rain forest and does not venture into the extensive adjacent dry forest with its many thousands of species of potential caterpillar and primary parasitoid hosts only a few hundred meters away. Indeed, there is a single record of Taeniogonalos woodorum (DHJPAR0016846) well into the microgeographic distribution of Taeniogonalos fasciatipennisDHJ02 (see below), emphasizing the parapatric nature of the distribution of these two species of Taeniogonalos. However, in remaining restricted to rain forest, it is representative of the thousands of other species of ACG Lepidoptera, Hymenoptera, and Diptera which are faithful to their respective ecosystems, even at the time when the intense six month rainy season turns the adjacent dry forest in a very wet ecosystem.
http://species-id.net/wiki/Taeniogonalos_fasciatipennis
Figures 8–14This species is distinguished as follows: vertex black; mandible outer surface black, upper surface yellow, teeth reddish brown; clypeus with large yellow spot on each side; narrow yellow line on gena on hind orbit (Figs 8, 10); mesoscutellum entirely yellow or with thin medial black stripe; second metasomal tergum with broad posterior transverse band, not extended laterally (Figs 8, 10). Female armature present, in ventral view with lobes short and with shallow central depression (Fig. 12), in lateral view, posterior and ventral sides perpendicular, not directed downward at apex (Figs 8, 11); male genitalia with paramere long, about three-quarters length of gonostipes (Fig. 14); paramere in lateral view with dorsal margin straight (Fig. 13), without slight depression.
Taeniogonalos fasciatipennis was described by
The female syntype (not examined) from Veracruz was treated by
This and the species from North America and Cuba have long been regarded as the “gundlachii” group, the members of which are distinguished from others by having similar color and the presence of distinctive female armature. However, Taeniogonalos fasciatipennis can be distinguished from North American Taeniogonalos gundlachii by the female armature and male parameres as described above. Taeniogonalos fasciatipennis is morphologically separable from Taeniogonalos woodorum by the former having the female armature present and the male paramere short in relation to the gonostipes.
The DNA barcodes of Taeniogonalos fasciatipennisDHJ01 and Taeniogonalos fasciatipennisDHJ02 are 5.66% divergent from each other in the COI barcode region and both are 9% divergent from Taeniogonalos woodorum.
Mexico and Costa Rica.
Taeniogonalos fasciatipennisDHJ01 53, 42 barcoded; Taeniogonalos fasciatipennisDHJ02 15, 13 barcoded. Deposited in USNM, INBio, CNC, BMNH.
In the absence of genetic information, Taeniogonalos fasciatipennis appears to be one morphologically distinctive species that occurs throughout ACG dry forest (85 m to about 1300 m elevation) and does not occur in adjacent ACG rain forest or cloud forest. It is a hyperparasitoid of a wide variety of caterpillar and primary host species (see below). However, DNA barcoding has revealed that this morphologically-distinct species is divided into two distinct mitochondrial types in the ACG dry forest. One, baptized here as Taeniogonalos fasciatipennisDHJ01 (Figs 8, 9, 11–14) is an interim name in the inventory website database (http://janzen.bio.upenn.edu/caterpillars/database.lasso) and occurs throughout the ACG dry forest (85 to 700 m elevation). The other, interim name Taeniogonalos fasciatipennisDHJ02 (Fig. 10), has a very distinctive microgeographic distribution. Twelve of the 13 records are from the intermediate elevation southwest facing slope of the volcanic massif of Rincon de la Vieja (325–1276 m elevation in Sector Mundo Nuevo of ACG). The single other rearing record (two individuals from tachinid fly puparia from the same caterpillar) is from a site that is an ecogeographic analogue in biota, elevation, and climate to the Sector Mundo Nuevo site (600 m on the southwest facing slope of Volcan Cacao in Sector Cacao of ACG). Taeniogonalos fasciatipennisDHJ01 is probably the same as the lectotype from western (dry forest) Mexico, but since there is no present way to know for certain, both Taeniogonalos fasciatipennisDHJ01 and Taeniogonalos fasciatipennisDHJ02 have to be treated as interim names, and are therefore not italicized (and see below).
In general terms, Taeniogonalos fasciatipennisDHJ01 conspicuously ranges from the driest parts of ACG dry forest to its intermediate-elevation intersection with cloud forest and rain forest, and Taeniogonalos fasciatipennisDHJ02 is restricted to the upper, cooler, moister portion of this range. To emphasize the cooler and moister aspect of this very small range, there is even a single specimen (DHJPAR0016846) of the rain forest specialist, Taeniogonalos woodorum, from the very center of the range of Taeniogonalos fasciatipennisDHJ02. While Taeniogonalos fasciatipennisDHJ01 has not been found in the Sector Mundo Nuevo exact site occupied by Taeniogonalos fasciatipennisDHJ02, and thus they are parapatric, Taeniogonalos fasciatipennisDHJ01 is absolutely sympatric with the single record of Taeniogonalos fasciatipennisDHJ02 on the southwestern slopes of Volcan Cacao.
This situation creates two different taxonomic problems. First, since the two apparent ACG species within what appears morphologically to be Taeniogonalos fasciatipennis currently cannot be distinguished by any morphological trait, there is no way to know which of the two matches the lectotype from the State of Guerrero, Mexico. It is also possible that neither does. Second, the presence of two sympatric/parapatric species of “Taeniogonalos fasciatipennis” in the small area of ACG raises the spectre that this species of wasp may be a complex of sibling (cryptic) species. In contrast to parallel cases with extremely host-specific Microgastrinae Braconidae (e.g.,
If Taeniogonalos fasciatipennisDHJ02 were not distinctive by barcode from Taeniogonalos fasciatipennisDHJ01 (Fig. 2), it would not have been noticed. Taeniogonalos fasciatipennisDHJ01 and Taeniogonalos fasciatipennisDHJ02 have the same extremely varied lists of caterpillar and primary parasitoid hosts. In brief, Taeniogonalos fasciatipennisDHJ01 has been reared and barcoded 48 times from nine Lepidoptera families: Crambidae, Hesperiidae, Megalopygidae, Noctuidae, Nymphalidae, Papilionidae, Saturniidae, Sphingidae, and Uraniidae. While 53 more specimens of Taeniogonalos fasciatipennis have been raised, until (and if) those specimens are successfully barcoded, we cannot categorize them as Taeniogonalos fasciatipennisDHJ01 or Taeniogonalos fasciatipennisDHJ02.
In all cases, the primary parasitoid host was Tachinidae: Acantholespesia, Belvosia, Blepharipa, Calolydella, Carcelia, Chetogena, Drino, Eucelatoria, Hemisturmia, Houghia, Hyphantrophaga, Leschenaultia, Lespesia, Patelloa, Winthemia, and Zizyphomyia. If we add to this the other specimens of “Taeniogonalos fasciatipennis” that were not successfully barcoded but occupy the ACG ecosystem occupied by Taeniogonalos fasciatipennisDHJ01 a few more Lepidoptera families and tachinid genera are added to these lists, as well as three large-bodied genera in the Ichneumonidae (Enicospilus, Pedinopelte, Trogus).
Taeniogonalos fasciatipennisDHJ02 has been reared 13 times from parasitoids of Crambidae, Hesperiidae, Noctuidae, Notodontidae, Nymphalidae, Riodinidae, Saturniidae, and Sphingidae. The primary host genera are Tachinidae (Blepharipa, Drino, Houghia, Lespesia, Patelloa), Ichneumonidae (Enicospilus) and Braconidae (Macrocentrinae).
http://species-id.net/wiki/Taeniogonalos_gundlachii
Figs 15–20This species is noted here because Costa Rican specimens of Taeniogonalos have been previously identified as belonging to this species.
The color of Taeniogonalos gundlachii (Figs 15–17) is very similar to Taeniogonalos fasciatipennis (Figs 8–10) from Costa Rica, but we noted several morphological differences which appear consistent in specimens examined: lobes on female armature on sternum 2 in ventral view much longer and central emargination deeper (Fig. 18) than in Taeniogonalos fasciatipennis (Fig. 12); female armature in lateral view more rounded, and slightly protruding ventrally (Fig. 17) than the squared appearance in Taeniogonalos fasciatipennis (Fig. 11); male paramere slightly indented dorsally (Fig. 19) rather than straight in Taeniogonalos fasciatipennis (Fig. 13).
Specimens from the northern parts of the range of Taeniogonalos gundlachii, northeastern United States and Canada, are relatively uniform in color, black with yellow maculation as in Figs 15–17. Specimens from Cuba, Florida, Louisiana, and Texas have a broader yellow band on the inner and outer orbits; legs all yellow with only coxae black; male with one yellow band on the metasoma, and female with 3–4 yellow bands. We have not seen specimens from the area between Texas and Guerrero, Mexico, and have seen only the type of Taeniogonalos fasciatipennis from Mexico and one specimen from El Salvador which appears to be Taeniogonalos fasciatipennis.
It is not our intent here to resolve the entire taxonomic problem and there is not enough material available from Cuba and intermediate ranges. Therefore, we continue to apply the name Taeniogonalos gundlachii to the specimens from Canada to Cuba, while suspecting that those from Canada and eastern U. S. eventually will again be called Taeniogonalos costalis. Though we cannot deny the possible presence of Taeniogonalos gundlachii in Costa Rica, the ACG dry forest specimens reared in this study are different from those from North America, and thus we refer them to Taeniogonalos fasciatipennis.
The DNA barcode for specimens from Virginia, West Virginia, and Mississippi is 8.6% divergent from Taeniogonalos woodorum and 7.49–7.75% divergent from Taeniogonalos fasciatipennisDHJ02 and Taeniogonalos fasciatipennisDHJ01, respectively.
Canada to Cuba, and west to Wisconsin and Texas.
200+; 25 DNA barcoded. Deposited in USNM.
See
| 1 | Predominately yellow with black maculations | Taeniogonalos ornata |
| – | Predominately black with yellow markings (as in Figs 4–6, |
2 |
| 2 | Vertex with two oblique yellow stripes; yellow on gena broad, extending dorsally to occiput (Figs 4, 5); anterior half or third of mesoscutellum yellow (Fig. 5); mandible mostly yellow; female armature absent (Fig. 6); male parameres short, about half length of gonostipes (Fig. 7); ACG rain forest | Taeniogonalos woodorum |
| – | Vertex black; yellow on gena confined to narrow stripe on hind orbit (Figs 8, 10); metascutellum mostly yellow; mandible mostly black; female armature present (Figs 11, 12); male parameres longer, about ¾ length of gonostipes (Fig. 14); ACG dry forest | Taeniogonalos fasciatipennisDHJ01 and Taeniogonalos fasciatipennisDHJ02 |
http://species-id.net/wiki/Trigonalys_championi
Figures 21, 22This strikingly large species is mostly black, shiny, with the propodeum and first metasomal segment contrastingly white; the forewing is darkly infuscated with only the extreme apex and posterior margin somewhat lighter (Figs 21, 22).
The Trigonalys championi DNA barcode is 13–17 % different from other Trigonalidae in the ACG.
Guatemala, Costa Rica (
5; 5 barcoded. Deposited in USNM, INBio.
This species has been reared just four times, always from ACG dry forest and its ecotone with rain forest, and always from the large primary parasitoid wasp Enicospilus monticola (Cameron) (Ichneumonidae, Ophioninae) parasitizing Oraesia and Gonodonta spp. (Noctuidae) feeding on Piper (Piperaceae), Annona (Annonaceae), or Cissampelos (Menispermaceae).
Trigonalys championi, female. 21 Lateral view 22 Dorsal view.
http://species-id.net/wiki/Trigonalys_maculifrons
Figure 23This species is mostly yellow with various black maculations on the head and body (Fig. 23). The specimens reared from Costa Rica resemble this species with very similar black markings on the head and mesosoma. The black on the metasoma, however, differs. It is possible this is species-level variation, but we do not have enough specimens to evaluate this, and it does not seem to justify the description of a new species. Trigonalys flavescens Bischoff 1951, described from Mexico, is similar, but it differs by the more weakly striped metasoma and lack of a triangular mark at the top of the gena.
As explained by
The Trigonalys maculifrons DNA barcode is 13–17% different from other ACG Trigonalidae.
Trigonalys maculifrons, female, dorsal view.
Costa Rica, Guatemala, Honduras, Mexico (
4; 4 DNA barcoded. Deposited in USNM, INBio.
This striking species has been reared just three times, all in 2001 and in ACG dry forest (Sector Santa Rosa), from caterpillars of Euscirrhopterus poeyi Grote (Noctuidae) feeding on Pisonia aculeata L. (Nyctaginaceae) and primary parasitized by Lespesia posticaDHJ01 (Tachinidae).
| 1 | Black, propodeum and first metasomal tergum yellow (Figs 21, 22); forewing darkly infuscate, especially anteriorly; pronotal collar not raised | Taeniogonalos championi |
| – | Predominately yellow with black maculations on head and body (Fig. 23); wings uniformly hyaline; pronotal collar abruptly raised | Taeniogonalos maculifrons |
We emphatically and gratefully acknowledge the support of the ACG parataxonomist team in finding and rearing these caterpillars, their parasitoids, and their hyperparasitoids, and Area de Conservación Guanacaste for preserving the forests in which they live, and the Guanacaste Dry Forest Conservation Fund, the Wege Foundation, and the University of Pennsylvania for funding portions of the research. This study was also supported by NSF DEB 0515699 to DHJ and by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant to MAS. Laboratory analyses on sequences generated since 2009 were funded by the Government of Canada through Genome Canada and the Ontario Genomics Institute (2008-0GI-ICI-03). We thank D. G. Notton, The Natural History Museum, London; J. Huber and H. Goulet, Canadian National Insect Collection, Ottawa, J. Wiley, Florida State Collection of Arthropods, Gainesville, FL, and N. M Schiff, U. S. Forest Service, Stoneville, MS, for loan of specimens. Michele Touchet, Systematic Entomology Laboratory, USDA, Washington, DC, helped with the images. We appreciate reviews of the manuscript by the following: P. Tripotin, Mont Saint-Aignan, France, and M. L. Chamorro, Systematic Entomology Laboratory, USDA, Washington, DC. USDA is an equal opportunity provider and employer.