(C) 2012 Laura C. Sarzetti. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of Trichothurgus, described herein, Trichothurgus bolitophilus sp. n., nests in dry horse manure pads in Chubut, Patagonia, Argentina. The simplest nests consisted of one main tunnel ending in a series of 2 cells without partitions between them. In the more complex ones up to 6 cells were connected laterally to the main tunnel. Nests showed signs of reutilization. The behavior of nesting in horse manure is described for the first time in bees.
Trichothurgus bolitophilus sp. n., nests , horse manure, Patagonia, Argentina
The Lithurgini is a small basal group of the Megachilidae distributed in tropical to temperate and semi-arid regions worldwide (
This contribution describes the nests of the new species Trichothurgus bolitophilus, in horse dung pads. It represents the first description of the use of dung pads for nesting by bees.
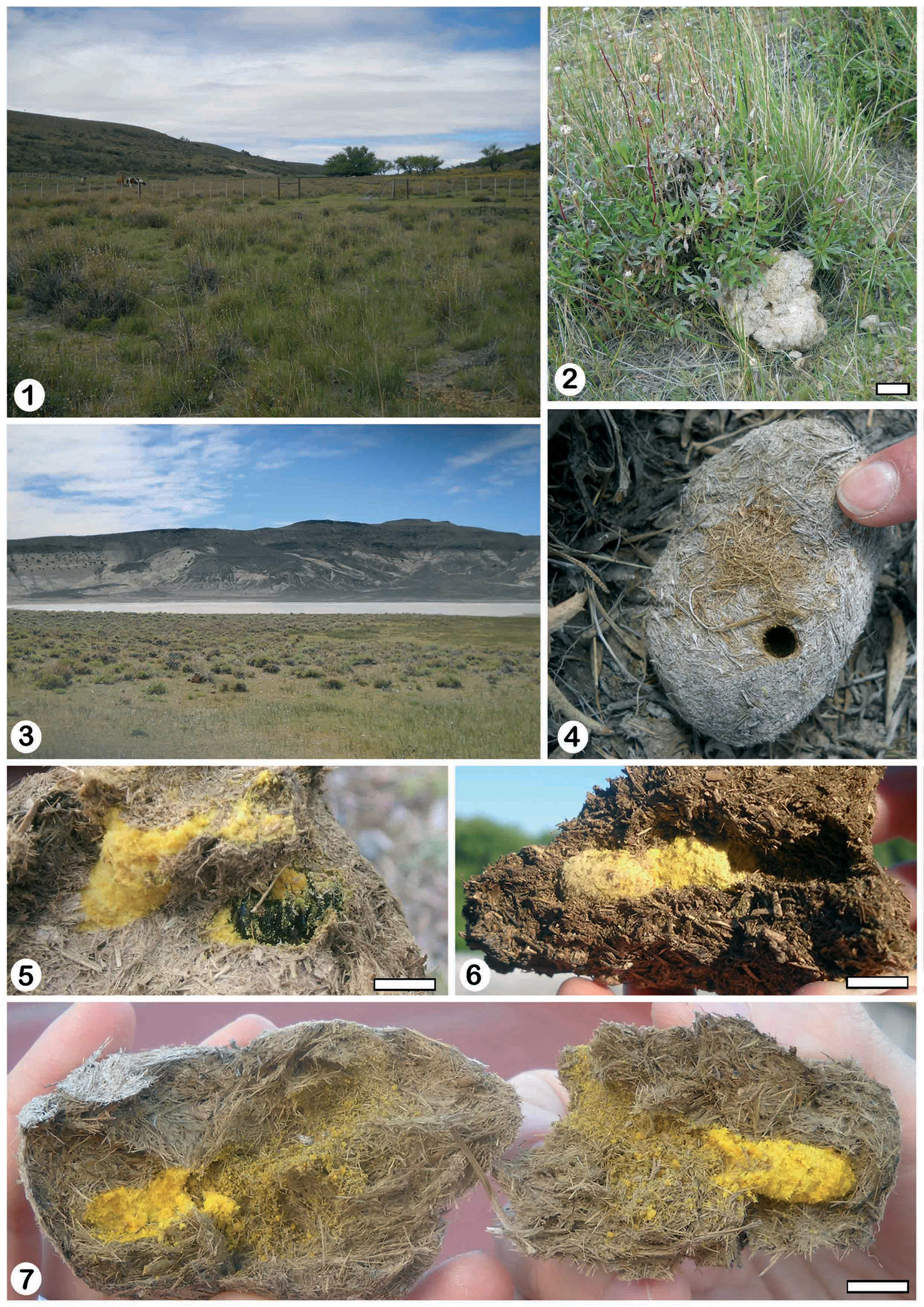
Study areaA total of 6 nests of Trichothurgus bolitophilus were found inside dry horse manure at two localities in Chubut Province (Patagonia, Argentina). Five nests were found at the Estancia Las Mellizas (locality 1) (45°04.91'S, 68°02.7'W; 589 m (Fig. 1) and one nest in “Laguna de los Flamencos” (locality 2) (44°37.58'S, 69°07.5'W; 526 m) (Fig. 3). Both localities are included in the driest zone of Chubut, where the annual rainfall ranges between 100 to 150 mm and the mean annual temperatures range between 8 and 11° C (
The nests were studied and collected in the field during 10 to 14 December 2010. At locality 1, dung pads with nests 1 to 5 were found within a fenced grassy area for horse grazing (Fig. 1). At locality 2, nest 6 was found in a pad near a small saline pond (Figs 3 and 4). The simplest nests consisted of one main tunnel ending in a series of 2 cells without partitions between them. Two of them also showed one short, empty, and blind tunnel. In the more complex ones up to 6 cells were connected also laterally to the main tunnel (Figs 6 and 7). Entrances of the nests were rounded and usually open (Fig. 4). The tunnels and cells were unlined, although they had a smooth surface (Fig. 7). The provisioned cells contained pollen of Asteraceae and Amaranthaceae in roughly equal proportions, and eggs or larvae. In some cases cells also contained remains of old cocoons, indicating reutilization of nests. Beyond these common features, each nest showed different morphologies that need to be described individually.
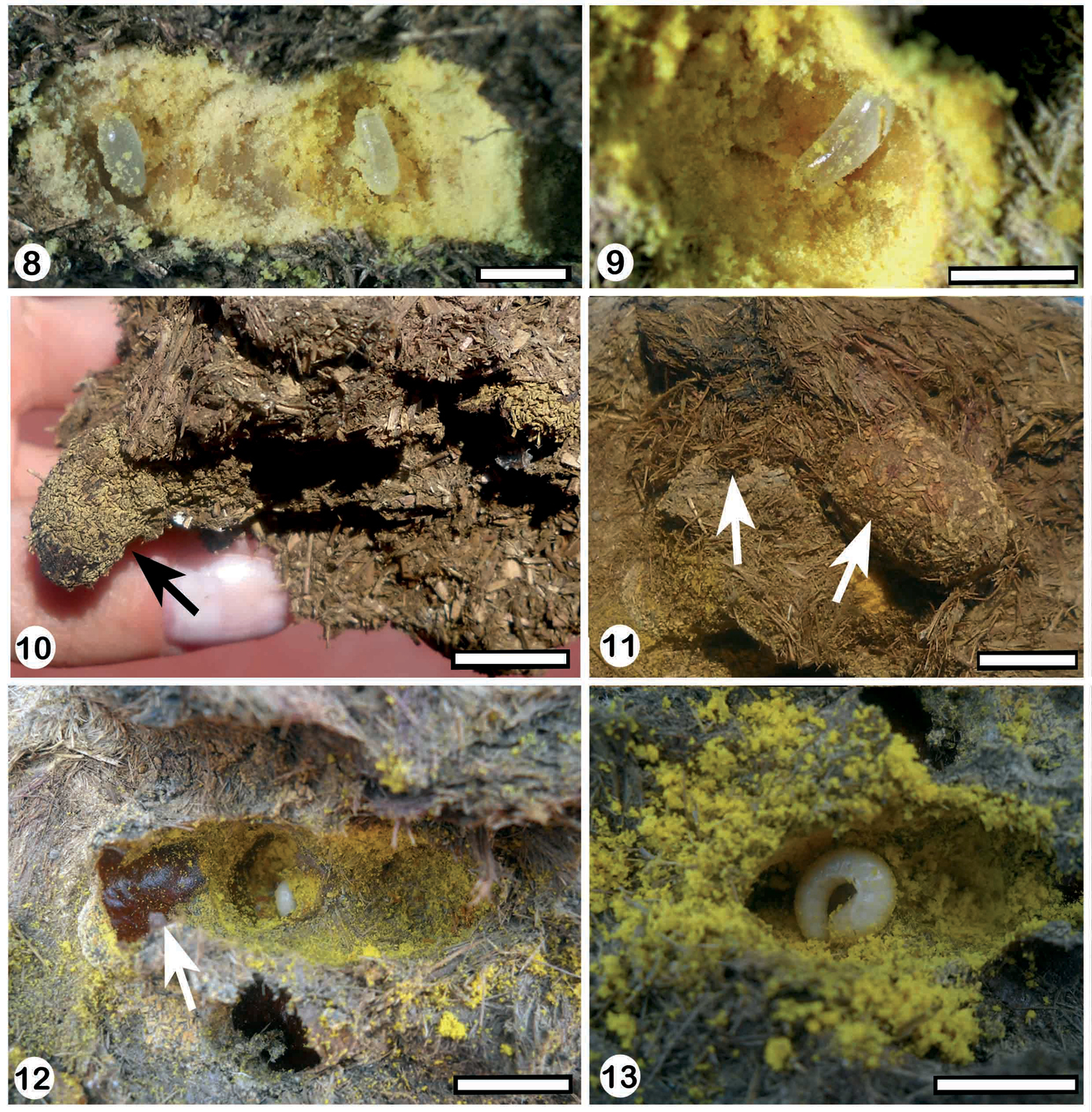
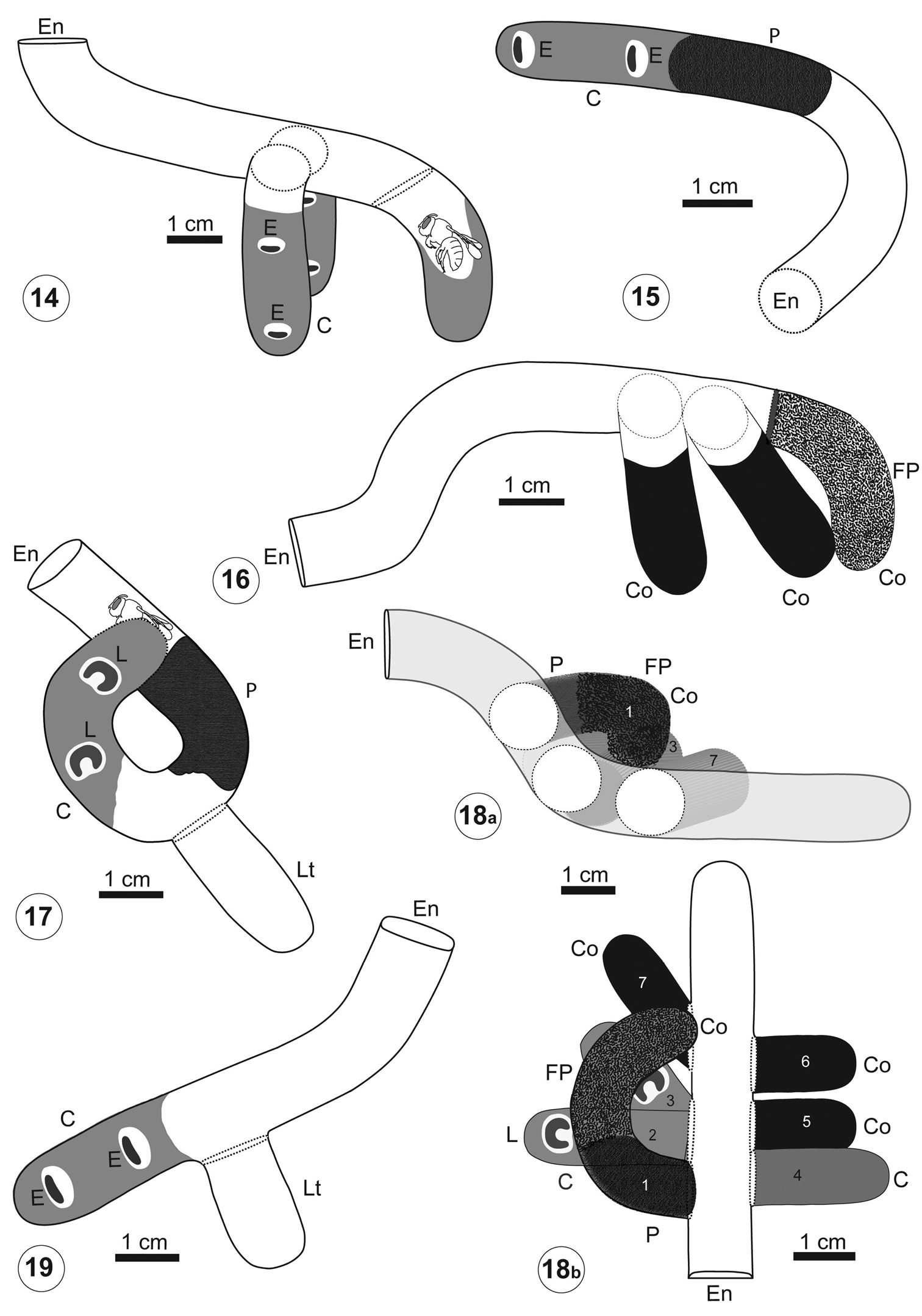
Nest 1 (Fig. 14): it was excavated in a piece of dung composed of two parts, a bigger one below (L: 11 cm, W: 15 cm, H: 9 cm) and a smaller part on top (L: 10 cm, W: 6 cm, H: 9 cm) (Fig. 2). The entrance was open and located in the middle of the upper part. The main tunnel was 6 cm long and 1 cm in diameter. Its first portion, located entirely in the upper piece of dung, was 4 cm long, vertical, and almost straight (Fig. 14). It continued into the other piece of dung in a short and horizontal portion, 2 cm long. At 5 cm from the entrance there were two opposite series of two cells, without partitions, directly connected to the tunnel (Fig. 14). The cells were vertical, with rounded ends, and closed by a dung disk that was concave outside. These two cells were 2.4 cm long and 1 cm in diameter. The cells were completely filled with yellow packed pollen that was moist, probably because of the mixture with nectar. The provision contained two eggs, each one was inside an ellipsoidal chamber slightly larger than the egg (Figs 8 and 9). The first and anterior egg was at 1.5 cm (N: 2) from the top of the packed provision. The distance between egg chambers was 10 mm (N: 2). The eggs were whitish translucent, 0.5 cm long and 0.15 cm wide, and cylindrical with rounded ends, and were located horizontally inside the chambers (Figs 8 and 9). The main tunnel ended in an open, incomplete, vertical third cell that had an alive, immobile female (Figs 5 and 14).
Nest 2 (Fig. 15): it was constructed in one piece of dung 8 cm long, 9 cm wide, and 5.5 cm high (Fig. 6). The entrance was open and located in one side of the pad. The nest showed one curved and horizontal main tunnel, 4.5 cm long and 1 cm in diameter that ended in a series of two cells without a partition between them (Fig. 15). The series of two cells was also horizontal, 2.9 cm long and 1 cm in maximum diameter and closed with a plug of dung fibers of 2.5 cm thick (Figs 8 and 15). The series of cells was provisioned with yellow packed pollen and contained two eggs which were included in individual chambers separated by 0.7 cm from each other. The dung pad also contained old cocoon remains without apparent connections with the tunnels. The cocoons were composed of a thin coriaceous layer of dark brown silk with a varnish-like substance on the smooth inner surface.
Nest 3 (Fig. 16): it was located in one piece of dung 11.2 cm long, 6.6 cm wide, and 4 cm high. The entrance, located laterally in the dung pad, was closed with a thin plug of dung fibers. The main tunnel, 6 cm long and 1 cm in diameter, was sinuous and ended in a cell containing cocoon remains (Fig. 16). The nest had two other old open cells with emerged cocoons inside. The cells were 2.5 cm long and 1 cm in maximum diameter. The cocoons were shorter than cells (2 cm long) and their walls were similar to those from nest 2 (Fig. 10). The first cell was located in the main tunnel at 6 cm from the entrance and it was vertical, with its bottom pointing downwards. The second cell was located beside the first one, with their bottom pointing laterally. The third, curved cell was located at the end of the main tunnel. The cocoons from the second and third cells had old fecal pellets attached to their walls. The fecal pellets were elliptical yellow-brown, and oval in cross section, with rounded ends. They were 0.9 mm long, 0.4 mm wide, and distributed randomly (Fig. 10, arrow).
Nest 4 (Fig. 17): it was located in a piece of dung 24.5 cm long, 18.4 cm wide, and 7 cm high. Theentrance was located up and laterally in the pad. At 1 cm from the entrance there was a dead female inside the main tunnel. The main tunnel was coiled, 4.6 cm long and 1 cm in diameter that was partially filled with dung fibers. At 4.5 cm from the entrance, it was an empty, short lateral tunnel. The main tunnel ended in an open and curved series of two cells without a partition between them. The series of cells, 2.5 cm long and 1.0 cm in diameter, contained yellow packed pollen and two young larvae.
Nest 5 (Fig. 18): it was located in a piece of dung 14.3 cm long, 8.4 cm wide, and 7 cm high. The main tunnel was horizonal, sinuous in vertical section, 6.7 cm long and 1 cm in diameter. At 2.5 cm from the entrance arose a C-shaped secondary tunnel, whose final portion rested over the main tunnel. The secondary tunnel was 4.4 cm long and 1 cm in diameter and contained remains of a cocoon (cell 1). The cocoon showed fecal pellets attached to its walls (Fig. 11, right arrow). The tunnel was closed with a plug of dung fibers (Fig. 11, left arrow). Adjacent to the secondary tunnel were located three more cells (cells 2-4). Cells 2 and 3 branched from the same point of the tunnel. Cell 2 was perpendicular to the main tunnel, whereas cell 3 was oriented in an acute angle to it. Cells 2 and 3 were 2.6 cm long and 0.8 cm in diameter. Both cells were located inside old cocoons (Fig. 12) and contained yellow packed pollen and a larva in each one. The larva from cell 2 was younger than that from cell 3 (Fig. 13). In addition, at 2.5 cm from the entrance and in front of the secondary tunnel was located a fourth cell (cell 4), inside an old cocoon. The cell 4 was 2.5 cm long, 0.8 cm in diameter, and contained packed pollen that seemed to be old because of its dryness and decoloration. At the sinuous, middle part of the main tunnel, were located three more cells (cells 5-7). Two of them (cells 5 and 6) at one side were straight and perpendicular to the main tunnel. Both cells were 1.5 long, 0.8 cm in diameter, and were empty, containing only old emerged cocoon. Cell 7, at the other side, was oriented at an angle to the main tunnel and was 2.5 long and 0.8 mm in diameter. It was also empty and contained only emerged old cocoon.
Nest 6 (Fig. 19): it was located in a piece of dung 8 cm long, 5.6 cm wide, and 4 cm high (Figs 4 and 7). The entrance was open and located on top. There were dung fibers and pollen grains on the dung around the entrance (Fig. 4). The main tunnel, slightly curved and inclined downwards, was 5.9 cm long and 1 cm wide finishing in an open series of two cells without partitions. The wall of the main tunnel had pollen grains adhering that gave it a yellow appearance. At 3.2 cm from the entrance arose an open and empty lateral tunnel. It was 1.9 cm long and 1 cm wide, with a rounded end (Fig. 19). At the end of the main tunnel was a series of two cells of 2.5 cm long and 1cm in diameter. The series of cells had packed pollen and two eggs, located in individual chambers 1.5 cm apart.
1 general view of locality 1 Estancia Las Mellizas 2 nest 1 at Estancias Las Mellizas 3 general view of locality 2 “Laguna de los Flamencos” 4 nest 6 showing the open entrance hole and dung fibers 5 nest 5 showing the third cell with an alive and immobile female 6 nest 2 showing the provisioned cells with yellow packed pollen 7 unlined tunnels and cells with pollen of nest 6. Scale lines: Fig 2: 10 cm, Figs 5–7: 1 cm.
8 the provisioned cells of nest 6 showing the eggs inside cavities 9 one egg inside the cavity of nest 6 10 third cell of nest 3 showing the fecal pelletsattached to the walls 11 cell 1of nest 5 showing the plug of dung fibers (left arrow) and the randomdistribution of fecal pellets in cocoon walls (right arrow) 12 old cocoon remainsinside cell 2 of nest 5indicating reutilization of cells 13 cell 2 of nest 5 showing young larvae. Scale lines: Figs 8–9: 0.5 cm, Figs 10–13: 1 cm.
Architecture of Trichothurgus bolitophilus nests 14 nest 1 15 nest 2 16 nest 3 17 nest 4 18a–18b nest 5, the numbers 1 to 7 indicate the cells and cocoons 19 nest 6. C: provisioned cell, Co:cocoon, E: egg, En: entrance, FP: fecal pellets, L: larva, P: plug of dung fibers. Scale lines: Figs 14–19: 1 cm.
Trichothurgus bolitophilus shares with most Lithurgini several behavioral characters. However, one important difference is striking: the substrate used for nesting. Typically, members of Lithurgus nest in dead, soft to relative hard dry wood or cactus (
Trichothurgus bolitophilus excavates its nests in horse dung. It is difficult to understand the advantages to nest in dung pads on Patagonian plateaus. Cows are absent at this altitude so the only providers of this type of dung are horses, which are scarce and restricted to house surroundings. They were introduced in this continent in historical times, all of which suggest that Trichothurgus bolitophilus must have had, and still has, alternative nesting substrates, which probably are similar in texture to dung. Other plant substrates used by Lithurgini are strong candidates. Cactaceae are present in the region, although these plants are also scarce and small. Dry cushions of Azorella monantha (Araliaceae) form a structure similar in aspect and texture to dung pads. Also wood from houses and fence posts may be alternative substrates in historical times. However, we failed to find nests in these substrates. Beside scarcity, exposed dung is subject to harsh environmental conditions. Strong winds are very common in Patagonia, which can blow easily dung pieces such as where nest 6 was found. In winter, these plateaus are covered with snow and the cold would seem to be too intense for the overwintering imagos or post-defecating larvae to be protected only by a centimetric layer of dung on the soil surface.
Typically, members of Lithurgini are considered to be oligolectic bees (
In most Lithurgini the cells are arranged in series (
Lithurgus chrysurus, Lithurgus collaris, Lithurgus huberi and Microthurge corumbae all, at least occasionally, reutilize their nests (
urn:lsid:zoobank.org:act:21847924-C4A8-4A78-8501-E252DC0EB426
http://species-id.net/wiki/Trichothurgus_bolitophilus
Figs 20, 24Female holotype. Body length 13.6 mm (paratypes, 13.0-16.0 mm), length of forewing 9.2 mm, maximum width of head 5.9 mm, maximum length of head 4.5 mm. Coloration.Integument black except under surface of flagellum and tibial spurs dark bseriesn; front tibial spur and claws dark ferruginous (later black apically). Wings evenly weakly infuscate, with apex appearing darker, due to black papillae; veins and pterostigma blackish.
Pubescence. Black, with yellowish hairs as follows: on vertex, between ocelli, dorsal portion of pronotum including pronotal lobe, scutum, scutellum, axilae and metanotum. Hairs on T1-T4 black at sides and dark brown medially, on T2-T5 forming black apical bands; T6 with dense covering of coarse, black hairs. Scopa black.
Punctation.Integument generally coriaceous, except smooth and shiny on labrum, clypeus, mandible, supraclypeal area below protuberance, around lateral ocellus, malar area, and on center of scutum. Clypeus with punctation sparse and irregular on basal medial area; punctures becoming smaller and denser toward apical and lateral margins. Supraclypeal area with few, scattered punctures below protuberance. Rest of head with small and dense punctures, except around ocelli. Mandibles unpunctured except on outer interspace. Scutum on mid-posterior region with large punctures separated by 0.3-1 times their diameter; punctures becoming smaller and denser on rest of surface; anteriorly with dense, poorly defined punctures. Scutellum and axilla with punctures separated basally, and denser toward posterior margin. Metapostnotum microsculptured. T1 with sparse, shallow punctures apically, separated by twice their diameter; punctures on T2-T5 becoming denser toward apex of metasoma.
Structure. Inner margins of eyes straight, subparallel, slightly divergent below (upper to lower interocular distance 0.97; paratypes 0.95-1.01); paraocular carina present. Lengths of scape, 1.20; pedicel, 0.24; flagellomeres 1 to 3, 0.42: 0.22: 0.22; flagellomere 10, 0.30; first flagellomere shorter than combined lengths of flagellomeres 2+3. Interantennal distance longer than distance from antennal insertion to median ocellus (1.10-0.50), shorter than antennocular distance (1.10-1.35), and subequall to antennoclypeal distance (1.10-1.00). Labrum 1.07 times as long as basal width (paratypes 1.03-1.35); base of labrum with median longitudinal keel (0.11 times length of labrum; variable in paratypes, 0.08-0.28, Fig. 23, k) short paramedian carina (Fig. 23, p) and strong lateral carina (Fig. 23, l); median keel continued apically by deep longitudinal furseries (Fig. 23, f) reaching preapical constriction (Fig. 23, c) (width of furseries 0.60 times median ocellar diameter; paratypes 0.74-0.88); median part of labrum broadened, with convex lateral margins (maximum width of median part 3.7 times median ocellar diameter; paratypes, 3.57-3.94); apex beyond constriction laterally pointed, and apically rounded to weakly pointed (some paratypes) (constriction as wide as median ocellar diameter; paratypes, 0.79-1.11). Clypeus flat, 0.73 times as long as basal width (paratypes 0.62-0.68); apical margin medially straight (Fig. 22). Supraclypeal area with facial protuberance prominent, convex in dorsal view, not carinate, laterally with conical projections (these projections are more developed in larger specimens) (Figs 21-22). Median ocellus located below supraorbital line; proportion of interocellar distance to ocellocular distance 0.64 (paratypes, 0.54-0.55); proportion of interocellar distance to ocelloccipital distance, 0.54 (paratypes, 0.46-0.50). Gena broader than eye in lateral view (1.17; paratypes, 1.25-2.66).
This species is closely related to Trichothurgus laticeps (Friese) and Trichothurgus dubius (Sichel) by the shape of the labrum, with a preapical constriction, and the similar shape of the facial prominence, with short lateral conical projections. It is intermediate in the color pattern of the vestiture, being Trichothurgus laticeps entirely black, and Trichothurgus dubius extensively white. The base of the labrum and the shape of the apical portion are different in the three species. The apex beyond the constriction is rounded in Trichothurgus laticeps and Trichothurgus dubius, without lateral conical projections. The base of the labrum in Trichothurgus dubius bears a bifid projection, while in Trichothurgus laticeps it bears a spiniform projection and several rugae between the median keel and the paramedian carinae.
A single male with the same labels as the female from Patagonia, San Jorge, is tentatively associated. Diagnostic structures of the labrum and the face in Trichothurgus are different in females and males, making sex association very difficult. This male has a similar color pattern, except that it bears white hairs on the face and on the first tergum. It completely lacks a facial protuberance, being the supraclypeal area slightly convex. Both the supraclypeal area and the clypeus are densely micropunctate, with the punctures coalescent, giving a dull appearance (Fig. 24). The labrum has a basal transverse depression delimited by a carina, the apex is rounded, and the median longitudinal depression is shallow (Fig. 24). The base of the labrum in Trichothurgus laticeps and Trichothurgus dubius is depressed laterally only, bearing medially a rounded protuberance which is not carinate apically. Males of Trichothurgus laticeps are further differentiated by the sparser punctures with shiny interspaces on the clypeus and supraclypeal area.
The name refers to the habits of this species, which makes its nests in dung pads (boliton, greek).
Argentina, provinces of Santa Cruz, Chubut and Mendoza.
Argentina. Holotype ♀: Chubut, ruta 24 entre Sarmiento y Paso de Indios, 44°37'35.20S, 69°7'30.10W, 529 m s.n.m., XII-2010, Genise col. (MACN); 2 ♀ paratypes, Chubut, Pampa Pelada, 45°4'54.89S, 68°2'48.68W, 20-XII-2010, Genise col. (MACN); 1 ♀ paratype, Patagonia, San Jorge (the San Jorge Gulf occupies the southern part of the province of Chubut and the northern part of the province of Santa Cruz) (MACN); 1 ♂ (tentatively associated) Patagonia, San Jorge (MACN); 1 ♀ paratype, Santa Cruz, ruta 3, El Salado, 22-II-1980, Willink, Fidalgo, Dominguez & Claps col. (IFML); 1 ♀ paratype, Mendoza, Ruta Termas Sosneado km 42, 11-I-1980, Willink, Fidalgo, Dominguez & Claps col. (IFML).
Trichothurgus bolitophilus Durante & Roig Alsina, sp. n. 20 female holotype, habitus 21 female paratype Pampa Pelada, face 22 female paratype Pampa Pelada, detail of clypeus and facial prominence 23 female paratype Pampa Pelada, labrum: k, median longitudinal keel; p, paramedian carina; l, lateral carina; f, longitudinal furrow; c, preapical constriction 24 male, face.
We thank Liliana Seoane for the identifications of pollen, Daniel Speranza for helping to draw and interpret the architecture of the nests, Pablo Puerta for his assistance in the field and two anynymous reviewers and the editor for their comments on the manuscript. This is a contribution of PICT 1972 from the Agencia Nacional de Promoción Científica y Tecnológica of Argentina to Jorge F. Genise.