(C) 2013 Cornelis van Achterberg. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The species of the genus Hybrizon Fallén (Hymenoptera: Ichneumonidae: Hybrizoninae) from China are reviewed, with special reference to Hunan (South China). The genus Hybrizon and two species (Hybrizon flavofacialis Tobias, 1988, and Hybrizon ghilarovi Tobias, 1988) are reported for the first time from the Oriental region. The species known from the Palaearctic and Oriental regions are keyed.
Ichneumonidae, Hybrizon flavofacialis, Hybrizon ghilarovi, Lasius fuyi, Oriental, China, Hunan, koinobiont endoparasitoids, ant larvae, key
The small subfamily Hybrizoninae Blanchard, 1845 (= Paxylommatinae Foerster, 1862, Hybrizontinae of authors, “Hybrizonites” of
The subfamily is known only from the Holarctic region and we report for the first time two species of the genus from the Oriental part of China. There are only two reports of the genus Hybrizon from China (
The biology of the Hybrizoninae has been for long time uncertain, but recently oviposition has been documented by photographing and filming two different genera (
The collecting site is at the border of the Southeast Lake near Yuanjiang (N. Hunan) in the common reed (Phragmites australis (Cav.)) zone, with Oriental “Lasius fuliginosus” (= Lasius fuyi Radchenko, 2005; see
For references to genera and species of Hybrizoninae, see
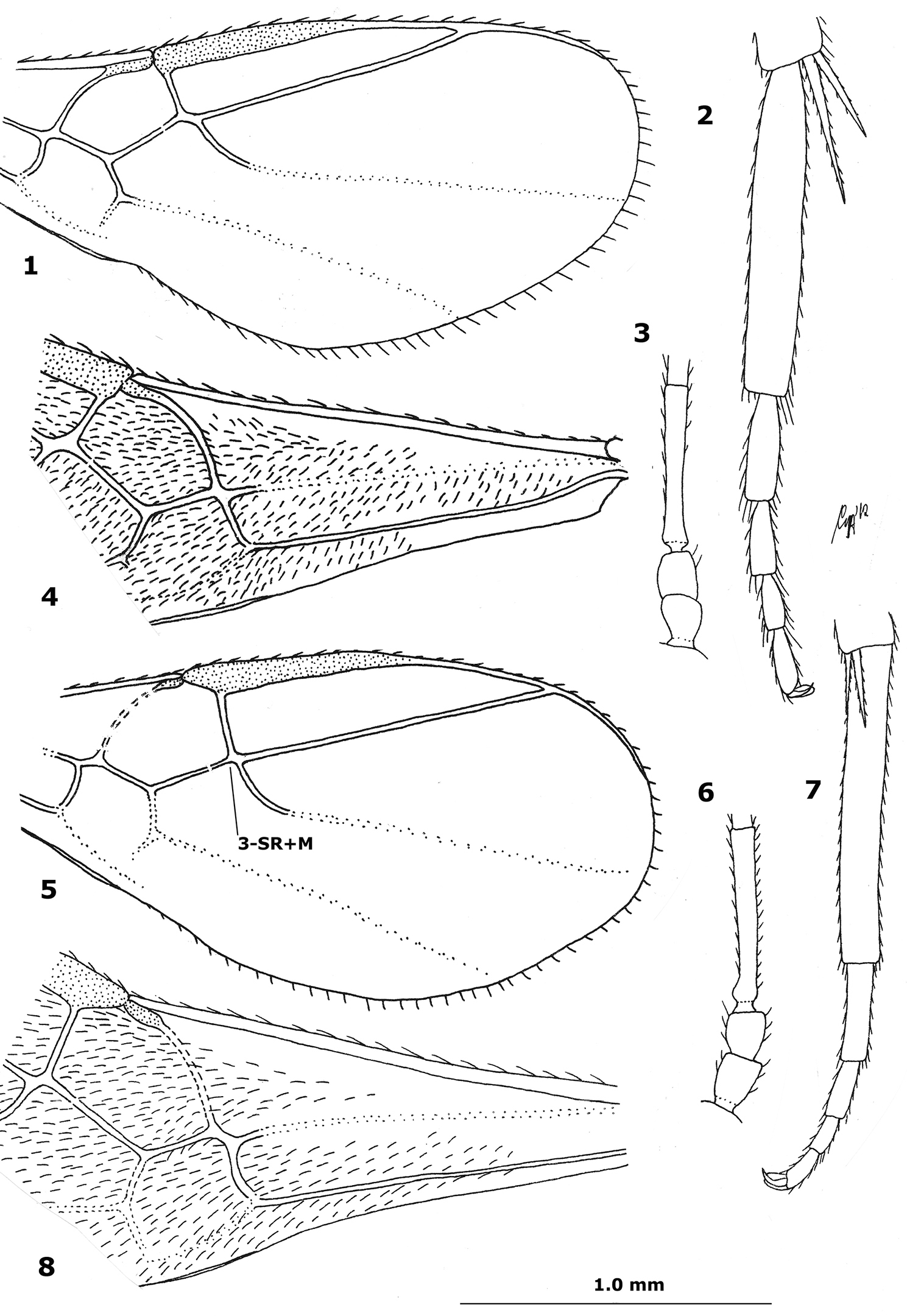
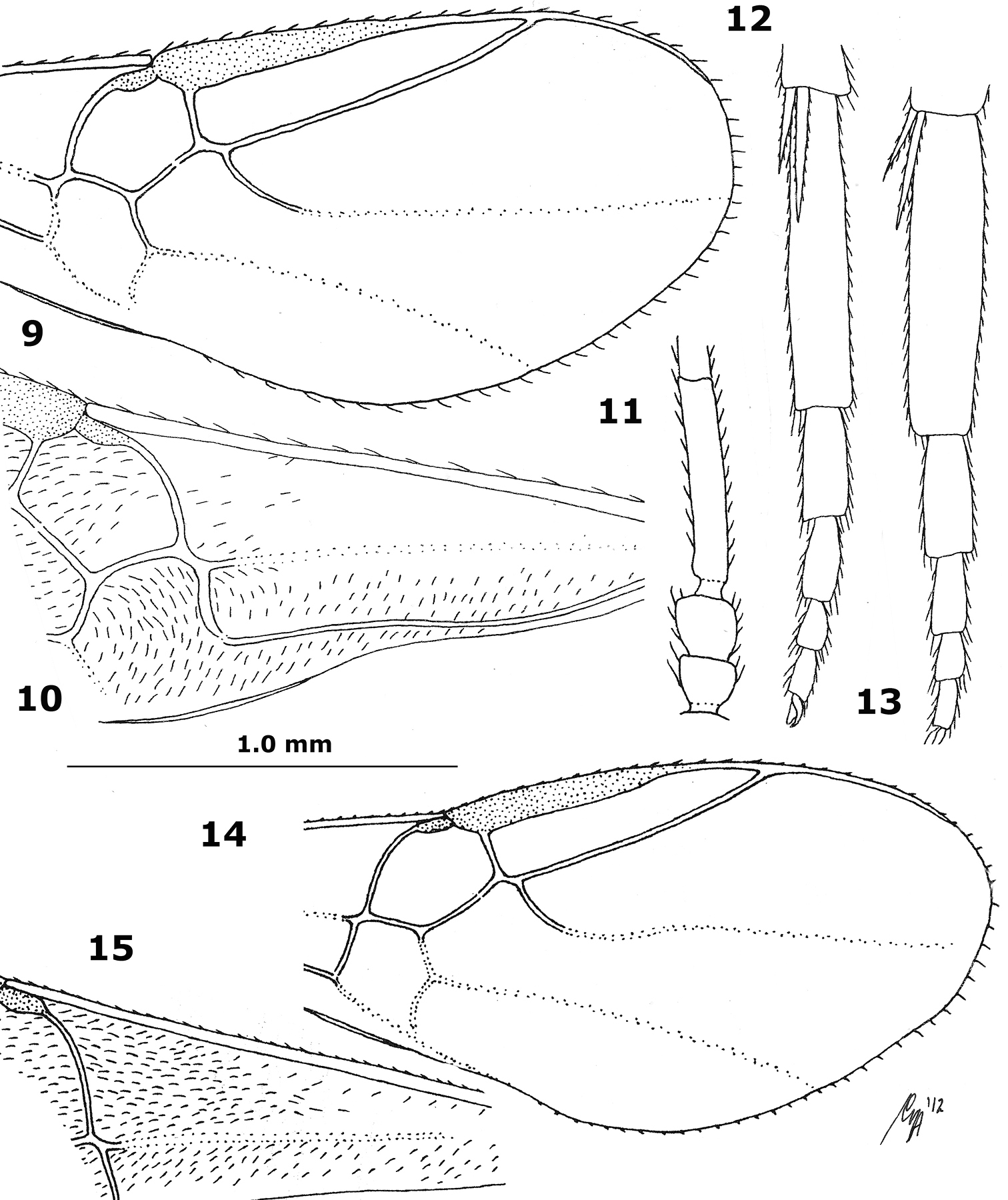
Figs 1–15
Hybrizon Fallén, 1813: 19 (no species);
Paxylomma de Brébisson, 1817: 66 (no species); Shenefelt, 1969: 2 (as synonym of Hybrizon Fallén, 1813);
Paxyloma Stephens, 1835: 119;
Paxylomme Wesmael, 1835: 88;
Paxyllomma Curtis, 1837: 115;
Paxylloma Blanchard, 1840: 335;
Pachylomma Ratzeburg, 1848: 53;
Plancus Curtis, 1833: 188;
Eupachylomma Ashmead, 1894: 58;
Reported from North China by He (1981: Heilongjiang, Jilin) and by
Basal cell of fore wing largely glabrous, with at most 15 setae (Fig. 10); scapus somewhat smaller than pedicellus (Fig. 11); third antennal segment comparatively stout (Fig. 11); ventral half of face and scutellum largely smooth; maximum width of face 1.4–1.5 times its minimum width; eyes glabrous; mesoscutum with pair of bands of distinct punctures, rarely punctures absent or obsolescent; scutellum (except sometimes laterally) and notaulic area of mesoscutum usually dark brown; propodeum largely smooth or granulate, except for medial carinae and posteriorly with weak or obsolescent curved carinae; vein 1-M of fore wing distinctly curved anteriorly (Figs 9, 10); vein r of fore wing issued comparatively close to base of pterostigma (Fig. 9); vein 1-M of fore wing paler than vein 2-CU1 of fore wing; in lateral view length of hind basitarsus 4–5 times its maximum width (Figs 12, 13); ventral half of metapleuron coriaceous; sparsely setose part of ovipositor sheath 0.2–0.3 times as long as second tergite; length of fore wing 2–3 mm.
3 ♀ +22 ♂ (HUNAU, RMNH), S. China: Hunan, Yuanjiang, Southeast Lakeside, Ben-Zhu Dai, together with Lasius “fuliginosus” (= Lasius fuyi Radchenko): 2 ♂, 3.VI.1989; 1 ♀ + 1 ♂, 4.VI.1989; 2 ♂, 8.X.1989; 11 ♂, 9.X.1989; 1 ♀ + 7 ♂, 10.X.1989; 1 ♀, 12.X.1989.
Face yellow; eyes glabrous; pedicellus wider and slightly longer than scapus (Fig. 3) and dark brown, contrasting with yellowish scapus; third antennal segment comparatively slender (Fig. 3); maximum width of face 1.2–1.3 times its minimum width; ventral half of face and scutellum more or less granulate; distance between posterior ocelli of female about 1.5 times diameter of ocellus (about twice in male); mesoscutum antero-laterally smooth; ventral half of metapleuron rugose or densely rugulose; posteriorly propodeum with strong curved carinae (but sometimes disappearing in rugosity); basal cell of fore wing (except basally) with 50–70 setae (Fig. 4); vein r issued at base of pterostigma (Fig. 1); vein 3-SR+M of fore wing medium-sized (Fig. 1); vein 1-M of fore wing weakly and gradually curved anteriorly or straight (Fig. 4); in lateral view length of hind basitarsus 6–7 times its maximum width (Fig. 2); sparsely setose part of ovipositor sheath 0.2–0.4 times as long as second tergite.
Up to now only known from the holotype from Far East Russia (Khabarovsk kray). The holotype is illustrated by
10 ♂ + 5 ♀ (HUNAU, RMNH), S. China: Hunan, Yuanjiang, Southeast Lakeside, together with Lasius “fuliginosus” (= Lasius fuyi Radchenko), Ben-Zhu Dai: 2 ♀ + 2 ♂, 10.X.1989; 1 ♂, 14.V.1989; 3 ♂, 25.V.1989, Lan-Shao You; 1 ♀, 4. VI.1989; 2 ♂, 8.X.1989; 2 ♀, 9.X.1989; 1 ♂, 11.X.1989; 1 ♂, 3.VI.1989.
Eyes distinctly setose; face dark brown, except near its tentorial pits; distance between posterior ocelli of female about 1.6 times diameter of ocellus; pedicellus about as wide as scapus and slightly shorter than scapus (Fig. 6), ventrally similarly yellowish coloured as scapus; third antennal segment comparatively slender (Fig. 6); maximum width of face 1.2–1.3 times its minimum width; ventral half of face and scutellum more or less granulate; area behind malar space flat or nearly so and rugose; scutellum granulate; propodeum areolate; ventral half of metapleuron largely rugose or rugulose; length of hind basitarsus about 7 times its maximum width (Fig. 7); mesoscutum antero-laterally rugulose; ventral half of metapleuron rugose or densely rugulose; vein r issued after base of pterostigma (Fig. 8); vein 3-SR+M of fore wing often short (Fig. 5); vein 1-M of fore wing weakly developed, straight anteriorly or nearly so (Fig. 5); basal cell of fore wing with 30–40 setae (Fig. 8); marginal cell of fore wing 4.0–5.5 times longer than its maximum width (Fig. 5); vein SR1 of fore wing straight (Oriental China) or sinuate (typical); posteriorly propodeum with strong curved carinae (but sometimes disappearing in rugosity); sparsely setose part of ovipositor sheath 0.6–0.7 times as long as second metasomal tergite.
1–4 Hybrizon flavofacialis Tobias, female, China, Hunan, Yuanjiang 5–8 Hybrizon ghilarovi Tobias, female, China, Hunan, Yuanjiang 1, 4 apical half of fore wing 2, 6 three basal antennal segments 3, 7 hind basitarsus lateral 4, 8 basal half of fore wing. 1 scale-line (= 1.0×); 2=1.7×; 3, 6–8=1.4×; 4, 5 =1.1×.
9–13 Hybrizon buccatus (de Brébisson), female, Bulgaria, Brodilovo, but 13 of female from Netherlands, Nunspeet 14–15 Hybrizon pilialatus Tobias, female, Italy, Funes 9, 14 apical half of fore wing 10, 15 basal half of fore wing 11 three basal antennal segments 12, 13 hind basitarsus lateral. 9 scale-line (=1.0×); 10–13=1.3×; 14, 15 from van
A female paratype is illustrated by
The Old World species can be separated as follows:
Key to Old World species of the genus Hybrizon Fallén
| 1 | Basal cell of fore wing largely glabrous, with at most 15 setae (Fig. 10); posteriorly propodeum with weak or obsolescent curved carinae; in lateral view length of hind basitarsus 4–5 times its maximum width (Figs 12, 13); vein 1-M of fore wing distinctly curved anteriorly (Figs 9, 10); third antennal segment less slender (Fig. 11); ventral half of face and scutellum largely smooth; ventral half of metapleuron coriaceous; maximum width of face 1.4–1.5 times its minimum width | 2 |
| – | Basal cell of fore wing (except basally) more or less setose (Figs 4, 8, 15); posteriorly propodeum with strong curved carinae (but sometimes disappearing in rugosity); in lateral view length of hind basitarsus 6–7 times its maximum width (Figs 2, 7); vein 1-M of fore wing weakly and gradually curved anteriorly or straight (Figs 4, 8, 15); third antennal segment comparatively slender (Figs 3, 6); ventral half of face and scutellum more or less granulate; ventral half of metapleuron rugose or densely rugulose; maximum width of face 1.2–1.3 times its minimum width | 3 |
| 2 | Vein r of fore wing issued comparatively far removed from base of pterostigma; mesoscutum without bands of punctures, at most with some punctures; vein 1-M of fore wing as dark as vein 2-CU1 of fore wing; scapus about as large as pedicellus; scutellum (except medio-anteriorly) and more or less notaulic area of mesoscutum ivory; length of fore wing 3.0–3.6 mm; propodeum distinctly rugose-granulate; Spain, South Korea | Hybrizon juncoi (Ceballos, 1957) |
| – | Vein r of fore wing issued comparatively close to base of pterostigma (Fig. 9); mesoscutum with pair of bands of distinct punctures, rarely punctures largely absent or obsolescent; vein 1-M of fore wing paler than vein 2-CU1 of fore wing; scapus somewhat smaller than pedicellus (Fig. 11); scutellum (except sometimes laterally) and notaulic area of mesoscutum usually dark brown; length of fore wing 2–3 mm; propodeum largely smooth or granulate, except for medial carinae; Northwest and East Palaearctic | Hybrizon buccatus (de Brébisson, 1825) |
| 3 | Eyes distinctly setose; pedicellus about as wide as scapus and about as long scapus (Fig. 6), ventrally similarly yellowish coloured as scapus; vein 1-M of fore wing straight anteriorly or nearly so (Fig. 8); sparsely setose part of ovipositor sheath 0.6–0.7 times as long as second metasomal tergite; vein 3-SR+M of fore wing often short (Fig. 5); East Palaearctic (Far East Russia); China (*Hunan, Jilin), South Korea, Japan (Hokkaido); Southeast Europe (Bulgaria) | Hybrizon ghilarovi Tobias, 1988 |
| – | Eyes glabrous; pedicellus wider and slightly longer than scapus and dark brown, contrasting with yellowish scapus; vein 1-M of fore wing weakly curved anteriorly (Figs 1, 14); sparsely setose part of ovipositor sheath 0.2–0.4 times as long as second tergite; vein 3-SR+M of fore wing medium-sized (Fig. 1) | 4 |
| 4. | Face yellow; vein r of fore wing issued at base of pterostigma (Fig. 1); distance between posterior ocelli of female about 1.5 times diameter of ocellus (but about twice in male); East Palaearctic (Far East Russia); *China (Hunan) | Hybrizon flavofacialis Tobias, 1988 |
| – | Face dark brown, except near its tentorial pits; vein r of fore wing issued distinctly removed from base of pterostigma (Fig. 14); distance between posterior ocelli of female usually about twice diameter of ocellus; West Palaearctic | Hybrizon pilialatus Tobias, 1988 |