(C) 2013 Christer Hansson. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Three morphologically very similar species of Asecodes Förster (Hymenoptera: Eulophidae) are reviewed. Asecodes parviclava (Thomson) is removed from synonymy under Asecodes lucens stat. rev., and differentiated from Asecodes lucens (Nees) and Asecodes lineophagum sp. n. All three species develop as gregarious endoparasitoids in larvae of Galerucella spp. (Coleoptera: Chrysomelidae), but each species has its own unique host range. Asecodes lineophagum attacks only Galerucella lineola (Fabr.) and Asecodes lucens only Galerucella sagittariae (Gyllenhal), whereas Asecodes parviclava parasitizes Galerucella tenella (L.), Galerucella calmariensis (L.) and Galerucella pusilla (Duftschmid). The Asecodes species are similar but display small though distinct morphological differences, and are distinguished also through molecular differences. The genetic distance in mitochondrial CO1 ranged from 2.3% to 7.3% between the species. Five names, one valid and four synonyms, were available for this group of species, but none of them was linked to a primary type. To promote stability of nomenclature, primary types are designated for all five names, neotypes for Eulophus lucens Nees, Entedon mento Walker and Derostenus parviclava Thomson, and lectotypes for Entedon chthonia Walker and Entedon metagenes Walker. Entedon mento, Entedon chthonia and Entedon metagenes remain synonymized under Asecodes lucens.
CO1, koinobiont endoparasitoids, host specificity, neotype designation, lectotype designation
Species of Asecodes Förster are described in the literature as parasitoids of chrysomelid beetles (
In this paper, we identify morphological traits that separate three species of Asecodes, corresponding also to molecular and biological data. We compared the morphotypes with previous descriptions and found that two correspond to described species, Asecodes lucens (Nees) and Asecodes parviclava (Thomson), whereas the third was not described. The new species is described under the name Asecodes lineophagum.
The colour photos were made with a Nikon SMZ 1000 stereomicroscope and a Nikon DS-5M camera. To eliminate reflections from the metallic and shiny body, a dome-light manufactured as described by
Morphological terms follow
The genetic distances were estimated from pairwise comparisons of 784 base pairs corresponding to the three prime end of CO1 (as used in Hambäck et al., unpublished). In the analysis, 7 individuals of Asecodes lucens, 23 individuals of Asecodes parviclava and 3 individuals of Asecodes lineophagum were used. The analysis was done in PAUP* ver. 4.0.a125 (
BMNH the Natural History Museum, London, United Kingdom (N. Dale-Skey Papilloud)
CH private collection of Christer Hansson
GNM the Natural History Museum, Gothenburg, Sweden (C.G. Jonsson)
LUZM Lund University Zoology Museum, Sweden (R. Danielsson)
NHRS the Natural History Museum, Stockholm, Sweden (H. Vårdal)
ZSM Zoologische Staatssammlung, München, Germany (S. Schmidt)
Five names were available for this complex of species prior to this investigation, with one name, Asecodes lucens, considered as valid and four other names as synonyms under the latter name. However, none of the names were fixed to a primary type and three of the names lacked type material altogether. For nomenclatural stability names must be fixed to a type specimen, and we therefore designate primary types, neotypes or lectotypes, for all five available names.
Eulophus lucens was described by Nees from a single female caught on Robinia pseudoacacia in Sickershausen [Bavaria, Germany] June 22, 1812, which was placed in his own collection. Specimens in the collection of Nees no longer exist apart from specimens sent to Westwood, now in the Oxford University Museum of Natural History (
Entedon mento was described by Walker from an unspecified number of males from near London and from Belfast. There is no material that agrees with the original description, either in the general collection or in the type collection of the Natural History Museum, London, where the material Walker based his descriptions is kept. In the type collection, the box supposed to contain some type material of Entedon mento is empty, but with a note “mento??”. Thus, it appears the material on which Walker based his description of Entedon mento is now lost. A female from England, Middlesex, Southgate, collected 6.vi.1972, fits the description of Entedon mento and is here designated as neotype for Entedon mento. The species was allegedly described from males, but males and females of this species are very similar. Further, Walker frequently misidentified the sex (
Derostenus parviclava was described by Thomson from an unspecified number of females he collected on Öland, an island in the Baltic Sea, and by G.F. Möller in Holmeja, a locality in Skåne, the southernmost province in Sweden. The collection of C.G. Thomson is in the Lund museum, and the collection of G.F. Möller is in the Natural History Museum in Gothenburg, both in Sweden. However, neither collection has any specimens from the type localities of Derostenus parviclava. There is a female under the name Derostenus parviclava in the G.F. Möller collection from Bökeberg (labeled “Bök”), which is a locality very close to Holmeja, one of the type localities. Because Thomson was very specific concerning localities from the province Skåne, where he lived and worked, it seems unlikely that he interchanged “Holmeja” with “Bökeberg”. However, the female from Bökeberg agrees well with the original description of Derostenus parviclava and it is from a locality very close to one of the original type localities. This specimen fulfills the criteria for a neotype for Derostenus parviclava and is designated as such here.
The descriptions of Entedon chthonia and Entedon metagenes do not have information on the number of specimens used, and neither has been fixed to a primary type. The type collection of the Natural History Museum, London, has a specimen each of Entedon chthonia (type no. 5.2603) and Entedon metagenes (type no. 5.2604). These specimens fit the original descriptions and are here designated as lectotypes.
The Asecodes species included here are gregarious koinobiont endoparasitoids of beetle larvae (
Parasitism rates may at times be very high, close to 100%, but may at other times be very low. In Sweden, where this study was performed, there seems to be a latitudinal shift in parasitism rates, at least for some hosts. The parasitism rates for Galerucella calmariensis and Galerucella tenella in northern localities, close to Umeå, are typically very high, between 50% and 100%, but less than 10% in more southern localities. The genus contains both strictly monophagous species and oligophagous species and the different Galerucella species often occur in the same localities, but on different wetland plants. There are often large differences in the parasitism rates between Galerucella species within the same locality. For instance, parasitism rates may be very high on Galerucella lineola and very low on other Galerucella species in one locality, whereas parasitism rates are high in another species in another locality. The different parasitism rates are not likely to be due to phenological differences or spatial distributions within localities because host plants colonised by different larval species may occur on neighboring plant individuals.
Genetic distances of Asecodes lucens vs Asecodes lineophagum calculated from mitochondrial gene data varied between 4.8–6.0 % (mean = 5.3%), Asecodes lucens and Asecodes parviclava 5.3–7.3 % (mean = 6.4%) and Asecodes lineophagum vs Asecodes parviclava 2.3–3.8% (mean = 3.0%). Variation was estimated as 2.6% for Asecodes parviclava, whereas no variation was found within Asecodes lucens and Asecodes lineophagum for the sampled individuals.
For identification of the species treated here the following additions can be made to the latest key to European species of Asecodes in
Couplet 9, replace “mento” with “11”, and include the following:
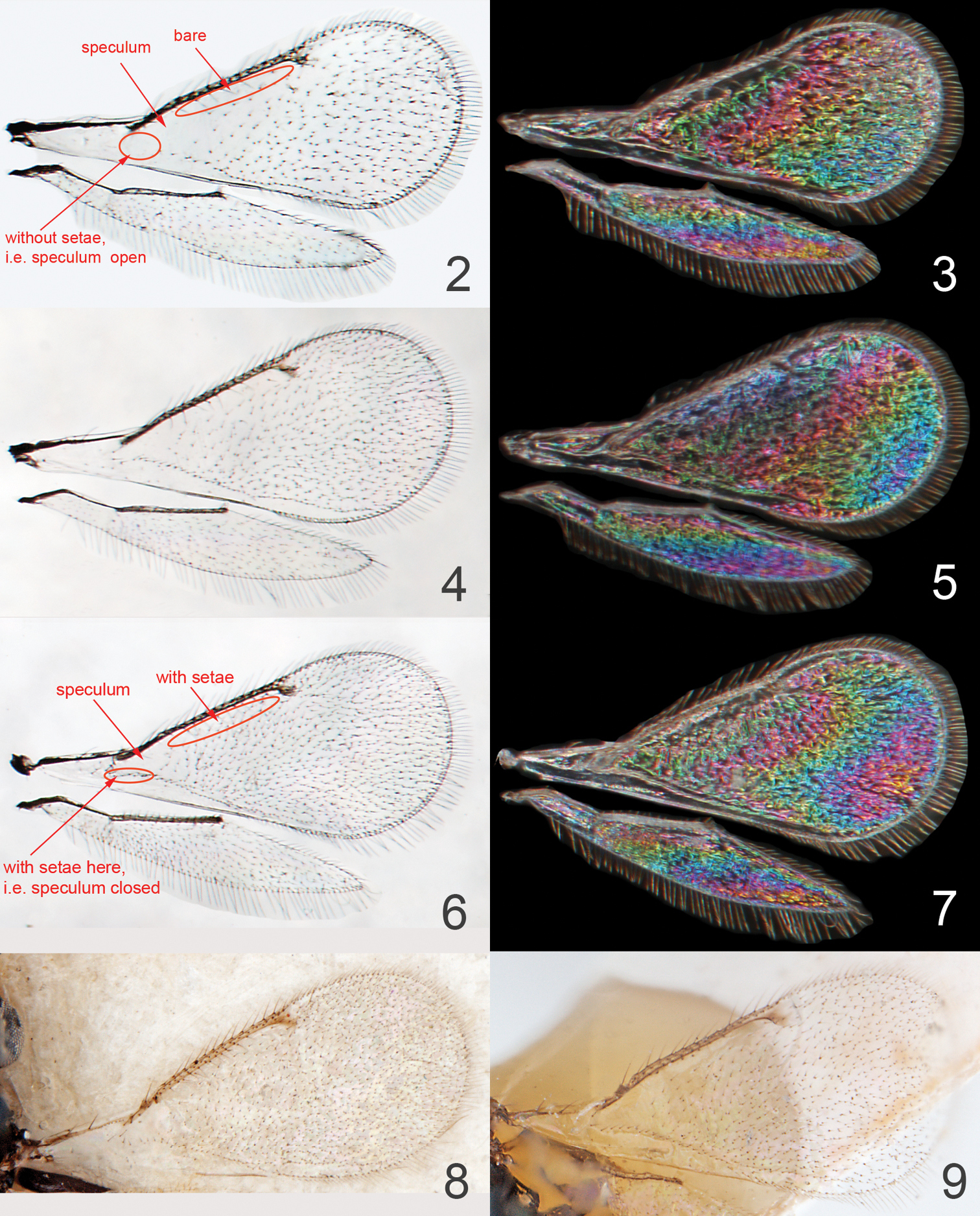
| 11 | Forewing speculum open (Fig. 2); propodeal callus with 3–5 setae (Fig. 22) | Asecodes lineophagum sp. n. |
| – | Forewing speculum closed posteriorly by costal setal line (Fig. 6); propodeal callus with 2 setae (Fig. 23) | 12 |
| 12 | Forewing bare just behind marginal vein, and relatively sparsely setose (Figs 4, 9, see also Fig. 2) | Asecodes parviclava (Thomson) |
| – | Forewing setose just behind marginal vein setose, and relatively densely setose (Figs 6, 8) | Asecodes lucens (Nees) |
urn:lsid:zoobank.org:act:3B302B7C-F323-45E3-8E78-19C080201E1A
http://species-id.net/wiki/Asecodes_lineophagum
Figures 2, 3, 10, 13, 16, 17, 22, 26, 27Forewing (Fig. 2) with speculum open posteriorly (i.e. setal line absent), bare just behind marginal vein and otherwise relatively sparsely setose; propodeal callus with 3–5 setae (Fig. 22).
FEMALE. Length 1.0–1.8 mm.
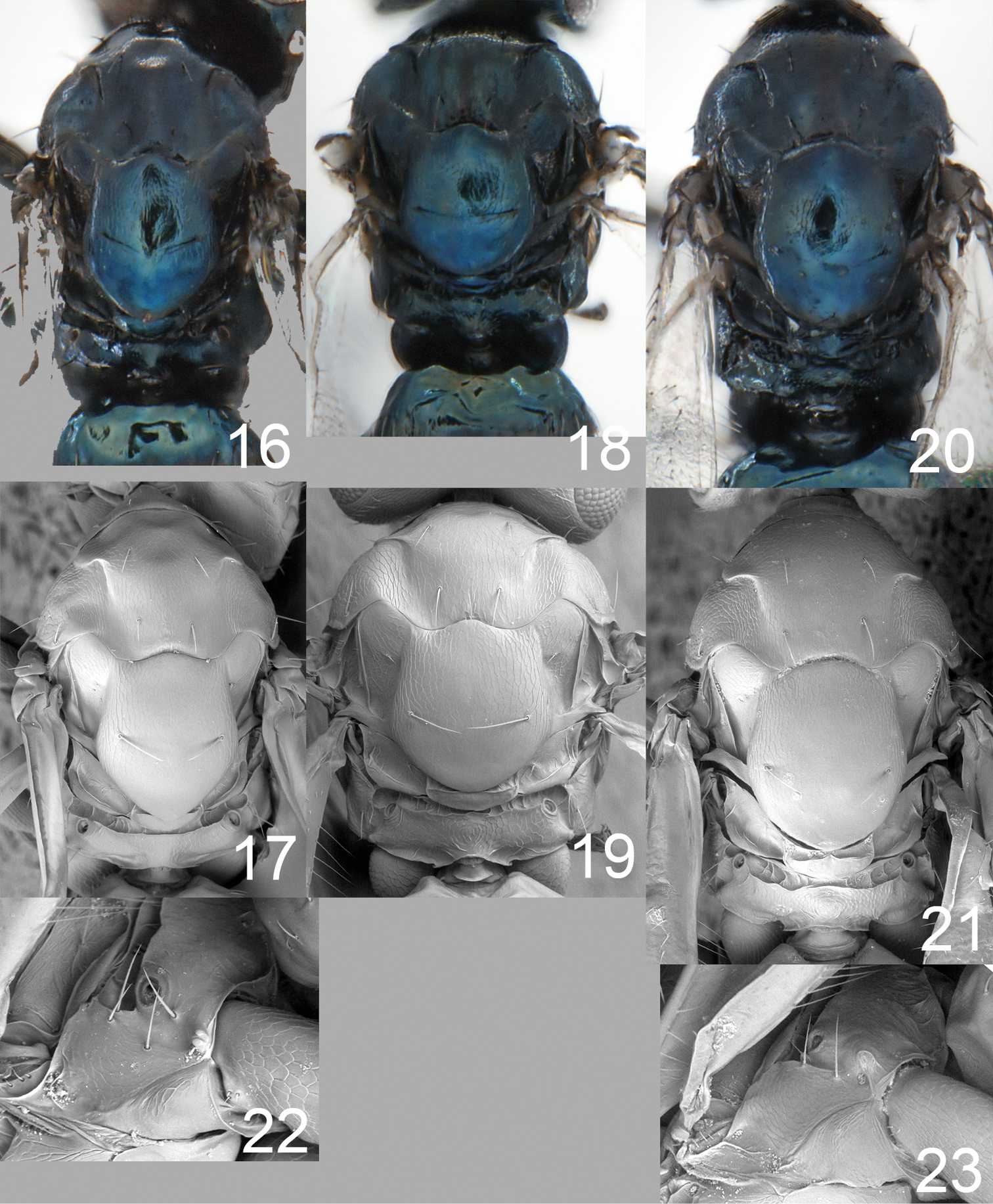
Antenna dark brown (Fig. 10). Frons below frontal suture metallic purple with upper-lateral corners close to eyes and frontal suture golden-green (Fig. 26), above suture golden green. Vertex metallic purple inside ocellar triangle, golden-green outside triangle. Mesoscutum black with metallic purple tinges (Fig. 16). Scutellum metallic bluish-green (Fig. 16). Axillae black with metallic purple tinges (Fig. 16). Dorsellum metallic bluish-green (Fig. 16). Propodeum metallic bluish-green (Fig. 16). Coxae, femora and tibiae dark brown to black, and shiny (as in Fig. 1); fore tarsus dark brown, mid and hind tarsi with tarsomeres 1–3 yellowish-white, tarsomere 4 dark brown. Forewing hyaline (Fig. 2), wing interference pattern as in Fig. 3. Petiole dark brown to black. Gaster with 1st tergite metallic bluish-green, remaining tergites dark brown to black with metallic purple tinges.
Antenna as in Fig. 10. Frons below frontal suture with weak reticulation, above suture smooth; antennal scrobes join on frontal suture. Vertex with very weak reticulation inside ocellar triangle, smooth outside triangle.
Mesoscutum with weak reticulation (Fig. 17). Scutellum with very weak reticulation in anterior 2/3 (Fig. 17), posterior 1/3 smooth. Axillae with weak reticulation (Fig. 17). Dorsellum slightly convex and smooth (Fig. 17). Propodeum with a wide groove along anterior margin (Fig. 17), with weak reticulation; propodeal callus with 3–5 setae. Forewing (Fig. 2) bare just behind marginal vein, speculum open, setation relatively sparse, and with 5–9 admarginal setae.
Petiole as a short, transverse, narrow stripe. Gaster circular.
Ratios. Height of eye/malar space/width of mouth = 2.6/1.0/1.8; shortest distance between posterior ocelli/posterior ocellus and eye/posterior ocellus and occipital margin = 10.4/5.4/1.0; width of head/width of mesosoma = 1.1; length of forewing/length of marginal vein/height of forewing = 2.4/1.0/1.0; length of postmarginal vein/length of stigmal vein= 0.6; length of mesosoma/length of gaster = 1.0.
MALE. Length 0.9–1.4 mm.
Very similar to female except antenna (Fig. 13) with scape wider, flagellomeres longer and more slender, and apical two flagellomeres distinctly separated.
Ratios. Height of eye/malar space/width of mouth = 2.1/1.0/1.6; length of mesosoma/length of gaster = 1.0–1.2.
All Swedish specimens were reared from Galerucella lineola (Coleoptera: Chrysomelidae) on Salix spp., mainly Salix cinerea. The sex ratio of each clutch on average is closer to one than for Asecodes lucens, and the standard deviation is distinctly higher in Asecodes lineophagum. Ratio female/male (n = 48): 3.10±2.68/2.27±2.17
HOLOTYPE female (BMNH) labelled “SWEDEN: Uppland, Ludden, 59°46'18"N, 18°40'19"E, 21.vi.2011, ex Galerucella lineola on Salix cinerea”. PARATYPES. 45♀ 49♂(BMNH, CH, LUZM, NHRS, ZSM): 25♀ 19♂with same label data as holotype; 3♀ 1♂“Sweden: Uppland, Fläktan, 59°46'54"N, 17°44'30"E, 24.vii.2011, ex Galerucella lineola on Salix cinerea.”; 3♀ 5♂“SWEDEN: Uppland, Liljekonvaljholmen, 59°48'18"N, 17°39'51"E, 18.vi.2011, ex Galerucella lineola on Salix sp.”; 6♀ 4♂“SWEDEN: Uppland, Mörtsjön, 59°38'39"N, 18°09"58"E, 5.vii.2011, ex Galerucella lineola on Salix cinerea”; 1♂“SWEDEN: Uppland, Sundängen, 59°33.954'N, 16°51.292'E, 25.vi.2011, ex Galerucella lineola on Salix cinerea”; 3♀ 6♂“SWEDEN: Uppland, Haknäs, 59°43'05"N, 17°41'57"E, 25.vi.2011, ex Galerucella lineola on Salix cinerea”; 1♀ “SWEDEN: Skåne, Skäralid, 6–17.viii.1994, M . Sporrong”; 3♀ (on two pins) labelled “Småland” [which is: SWEDEN: Småland, without further information]; 1♀ 1♂“SWEDEN: Skåne, Sövde, 5.vii.1985, C. Hansson”; 1♂“SWEDEN: Småland, Hyltebruk, 7–14.ix.1986, J. Ardö”; 1♂“NORWAY: Jostedalen, Gupne, 19.vii.1979, Hull University Expedition”.
http://species-id.net/wiki/Asecodes_lucens
Figures 6–8, 12, 15, 20, 21, 23, 24Forewing (Figs 6, 8) with speculum closed posteriorly by a setal line, setose just behind marginal vein and otherwise relatively densely setose; propodeal callus with 2 setae (Fig. 23).
All Swedish specimens were reared from Galerucella sagittareae (Coleoptera: Chrysomelidae) on Lysimachia thyrsiflora, Lysimachia vulgaris, and Potentilla palustris. The sex ratio of each clutch is female biased. Ratio female/male (n = 48): 4.27±1.83/0.92±0.94.
TYPE MATERIAL: Neotypes of Entedon lucens (ZSM) and Entedon mento (BMNH), lectotypes of Entedon chthonia (BMNH) and Entedon metagenes (BMNH), all types are females. ADDITIONAL MATERIAL: DENMARK: 4♀ 2♂(CH, LUZM). HUNGARY: Vas Co. 5♀ 2♂(ZSM). SWEDEN: Skåne 106♀ 13♂(swept) (BMNH, CH, LUZM); Uppland 205♀ 44♂(91♀ 25♂from Lysimachia, 114♀ 19♂from Potentilla palustris) (BMNH, CH, NHRS, ZSM); Öland 1♂(swept) (CH).
Figures 1, 4, 5, 9, 11, 14, 18, 19, 25
Forewing (Figs 4, 9) with speculum closed posteriorly by a setal line, bare just behind marginal vein and otherwise relatively sparsely setatose; propodeal callus with 2 setae (as in Fig. 23).
The Swedish specimens were reared from Galerucella calmariensis and Galerucella pusilla (Coleoptera: Chrysomelidae)on Lythrum salicaria, and Galerucella tenella on Filipendula ulmaria. The number of samples is smaller than for the other two species (n = 23, 10 from Galerucella calmariensis, 6 from Galerucella pusilla, 7 from Galerucella tenella). Ratio female/male: 2.09±1.16/0.91±0.95.
TYPE MATERIAL: Neotype female of Derostenus parviclava (GNM). ADDITIONAL MATERIAL: HUNGARY: Vas Co. 1♀ (BMNH); SWEDEN: Skåne 21♀ 16♂(CH, LUZM); Uppland 48♀ 21♂(24♀ 8♂from Galerucella calmariensis, 10♀ 8♂from Galerucella pusilla, 14♀ 5♂from Galerucella tenella) (BMNH, CH, NHRS); Västergötland 14♀ 5♂(CH, LUZM).
The separation of Asecodes lucens into three species based on molecular and morphological evidence is supported by biological data. Asecodes lucens and Asecodes lineophagum were reared from only one host species, Galerucella sagittariae and Galerucella lineola, respectively, whereas Asecodes parviclava was reared from three host species, Galerucella tenella, Galerucella calmariensis and Galerucella pusilla. These observations have also been confirmed with independent observations of parasitoid behaviour in the laboratory, where females were found to attack the respective host species, but not other species (L. Fors, unpubl. data). The delimitation of three species is also supported by observations in the field, where one species of Galerucella larvae may be heavily parasitized and another is not attacked in the same locality. Such parasitism patterns have, however, not been observed for Galerucella tenella, Galerucella calmariensis and Galerucella pusilla. In fact, earlier studies show strong correlations in parasitism rates between Galerucella tenella and Galerucella calmariensis among localities (
In view of our findings, the previous host records of Galerucella nymphaeae and Lochmaea suturalis for Asecodes lucens need confirmation. Investigation of Asecodes specimens reared from these hosts might quite possibly reveal additional cryptic species in this group.
Asecodes parviclava (Thomson), female habitus (length = 1.5 mm).
Asecodes spp., wings, females 2–3 Asecodes lineophagum sp. n. 2 transparent wings 3 wing interference pattern 4–5 Asecodes parviclava (Thomson) 4 transparent wings 5 wing interference pattern 6–7 Asecodes lucens (Nees) 6 transparent wings 7 wing interference pattern 8 Asecodes lucens, forewing, neotype 9 Asecodes parviclava, forewing, neotype.
Asecodes spp., antennae 10–12 females 10 Asecodes lineophagum sp. n. 11 Asecodes parviclava (Thomson) 12 Asecodes lucens (Nees) 13–15 males 13 Asecodes lineophagum 14 Asecodes parviclava 15 Asecodes lucens.
Asecodes spp. 16–21 thoracic dorsum, females 16–17 Asecodes lineophagum sp. n. 18–19 Asecodes parviclava (Thomson) 20–21 Asecodes lucens (Nees) 22–23 lateral propodeum in side view (anterior part to the left), female 22 Asecodes lineophagum 23 Asecodes lucens.
24–25 Asecodes spp., neotypes, females 24 Asecodes lucens (Nees) 25 Asecodes parviclava (Thomson) 26–27 Asecodes lineophagum sp. n., head in frontal view 26 female 27 male.
Thanks to museum staff (names listed under the museum acronyms above) for loan of type material, to the Department of Biology, Lund University, for the use of their SEM facility, and to Elisabeth Weingartner and Johannes Bergsten for assisting in handling the material. The study was funded by a grant from the Swedish Research Council Vetenskapsrådet (contract no. 621-2009-4943).