Journal of Hymenoptera Research 30: 13–18, doi: 10.3897/JHR.30.4018
Species status and new distribution records for
Lithurgus huberi Ducke (Hymenoptera, Megachilidae, Lithurginae)
Victor H. Gonzalez 1, Michael S. Engel 2, Mariano Lucia 3, Leopoldo J. Alvarez 3
1 Southwestern Oklahoma State University, Biological Sciences, 100 Campus Drive, Weatherford, Oklahoma, 73096, USA
2 Division of Entomology, Natural History Museum, and Department of Ecology & Evolutionary Biology, 1501 Crestline Drive – Suite 140, University of Kansas, Lawrence, Kansas, 66045, USA
3 División Entomología, Museo de La Plata, Universidad Nacional de La Plata, Paseo del Bosque s/n, 1900FWA, La Plata, Argentina. CONICET
Abstract
Notes are provided on the morphology of males and females of the enigmatic Lithurgus huberi Ducke (Lithurginae: Lithurgini), a species historically believed to have been introduced into South America from Asia and to be a possible synonym of the more widespread Lithurgus atratus Smith. Distinctive differences are documented between Lithurgus huberi and Lithurgus atratus, perhaps indicative of separate species. In addition, we provide new records of Lithurgus huberi in Argentina and Paraguay.
KeywordsAnthophila, bees, Apoidea, wood-boring bees
Introduction
Megachilid bees of the subfamily Lithurginae Newman are commonly known as wood-boring bees because they usually excavate burrows in dead, dry, often decayed wood (Michener 2007). The subfamily is unquestionable monophyletic and the sister group of Megachilinae (Roig-Alsina and Michener 1993, Engel 2001, Michener 2007, Gonzalez et al. 2012). Lithurginae consists of two tribes, Protolithurgini Engel, an extinct lineage preserved in mid-Eocene Baltic amber, and Lithurgini Newman, an extant taxon containing about 60 species in three genera: Lithurgus Berthold (currently with two subgenera), Microthurge Michener, and Trichothurgus Moure. Although the subfamily is relatively small in number of species, it is found on all continents except Antarctica. Only species of Lithurgus s.str. are found in the Eastern Hemisphere except for Lithurgus huberi Ducke, which occurs in Brazil and Argentina (Roig-Alsina 2006, Moure and Melo 2007). Snelling (1983) suggested that Lithurgus huberi was adventive to South America and probably a synonym of the Indo-Australian species Lithurgus atratus Smith given the morphological similarity between the two. Such a hypothesis is also supported by the wood-nesting habits of Lithurgus, which facilitates dispersion across great distances.
We confirmed the close morphological similarity between Lithurgus atratus and Lithurgus huberi, as noted by Snelling (1983). However, we also noted some morphological features in both sexes of Lithurgus huberi that seem to be consistent and that may prove to be useful in species recognition. Accordingly, the purpose of this note is to document and illustrate those characters, as well as record Lithurgus huberi for the first time for Paraguay and supplementing this with new distribution records for Argentina (vide infra).
Materials and methods
We examined (V.H.G. & M.S.E) the type series of Lithurgus atratus as well as of Lithurgus dentipes Smith, a species that has been considered a synonym of Lithurgus atratus, deposited in The Natural History Museum, London (NHML). Additionally, we examined 42 specimens of Lithurgus atratus from India, Celebes, Borneo, Solomon Islands, and Australia deposited in the Snow Entomological Collection (SEMC), Division of Entomology, University of Kansas Natural History Museum, Lawrence, Kansas, USA, and the U.S. National Pollinating Insects Collection, Bee Biology and Systematics Laboratory (BBSL), Utah State University, Logan, Utah.
Photomicrographs were prepared using a Canon 7D digital camera attached to an Infinity K-2 long-distance microscope lens, and were assembled with the CombineZMTM software package. Specimens are deposited in SEMC and the entomological collection of the Museo de La Plata, Argentina (MLP). Occurrence data for Lithurgus huberi were plotted using CorelDRAW® X5 and were taken from the literature (i.e., Ducke 1907, 1908, 1910, Camillo et al. 1983, 1994, Wittmann and Hoffmann 1990, Roig-Alsina 2006, Pick and Schlindwein 2011) as well as from specimen data retrieved from the Inter-American Biodiversity Information Network (IABIN) provided by the USDA, Agricultural Research Service Pollinating Insect Research Unit, Logan, USA, and the Coleção de Entomologia do Laboratório de Biologia Vegetal, Universidade Federal de Pernambuco, Recife, Brazil.
Results
Females of Lithurgus huberi primarily differ from those of Lithurgus atratus by the facial prominence. In Lithurgus huberi it is more strongly punctate and more depressed along the epistomal sulcus than in Lithurgus atratus (cf. Figs 1, 3 vs. 2, 4). Likewise, males of Lithurgus huberi differ from those of Lithurgus atratus in the inner glabrous surface of the metabasitarsus, which is broader and more projected (cf. Figs 5 vs. 6). Although subtle, such morphological differences appear to be consistent across the specimens examined. However, we have had access to a limited number of individuals from a restricted set of localities and cannot rule out that such differences represent mere geographic variations.
Despite the small number of species in Lithurgus s.str., the status of most of them remains questionable, particularly those from Southeast Asia. For example, at least eight ‘species’ that are closely related to Lithurgus atratus have been suggested to represent a single taxonomic unit (Michener 1965), although they could be a complex of rather cryptic species. Some of these are practically indistinguishable from Lithurgus atratus, differing mostly in body size (e.g., Lithurgus atratiformis Cockerell). Undoubtedly, a revision of the group that includes a great number of specimens from multiple locations (thereby necessitating extensive fieldwork as existing collections have significant gaps for Lithurgus across its distribution) is needed before any taxonomic action can be taken with certainty. Such a work would ideally be accompanied by molecular data to further test putative species boundaries as well as to elucidate possible routes and times of dispersion.
In conclusion, we support the view that Lithurgus huberi is closely related to the Indo-Australian Lithurgus atratus species or species group and, if dispersed by human activity, it might have reached South America at least 100 years ago when it was described (Ducke 1907). However, we show here consistent, albeit somewhat subtle, morphological features to distinguish Lithurgus huberi from Lithurgus atratus. For the time being we recommend that they should be treated as separate species.
Taxonomy
New records.
Argentina: 1♂, Buenos Aires, Berazategui (Parque Pereyra Iraola), 24-1-2011, Col. Alvarez. L-Lucia. M (MLP) (foraging on Ipomoea purpurea (L.) Roth); 1♀, Misiones, Loreto, February 1954 (SEMC). Paraguay: 2♀♀, 4♂♂, Itapua Vega, Dec 1955, Juan Foerster (SEMC).
Additional material examined.
Brazil: 3♀♀, 3♂♂, B. Horizonte, Minas, Brasil. A. Costa Jr. 20-4-49; 1♂, Para, Conceicão do Araguaia, July 1959, M. Alvarenga; 1♂, Paraíba, Santa Luzia, Mun. Serra do Brandão dos Chandoca, 4/8 December 1955, Sebastidos Madeiros (SEMC).
Figures 1–6.
Details of Lithurgus huberi Ducke from Minas Gerais, Brazil (left column: 1, 3, 5) and Lithurgus atratus Smith (NHML syntypes) (right column: 2, 4, 6). Facial views (1, 2), detail of facial prominence (3, 4), and inner surface of metabasitarsus emphasizing the apical expansion and glabrous surface (5, 6).
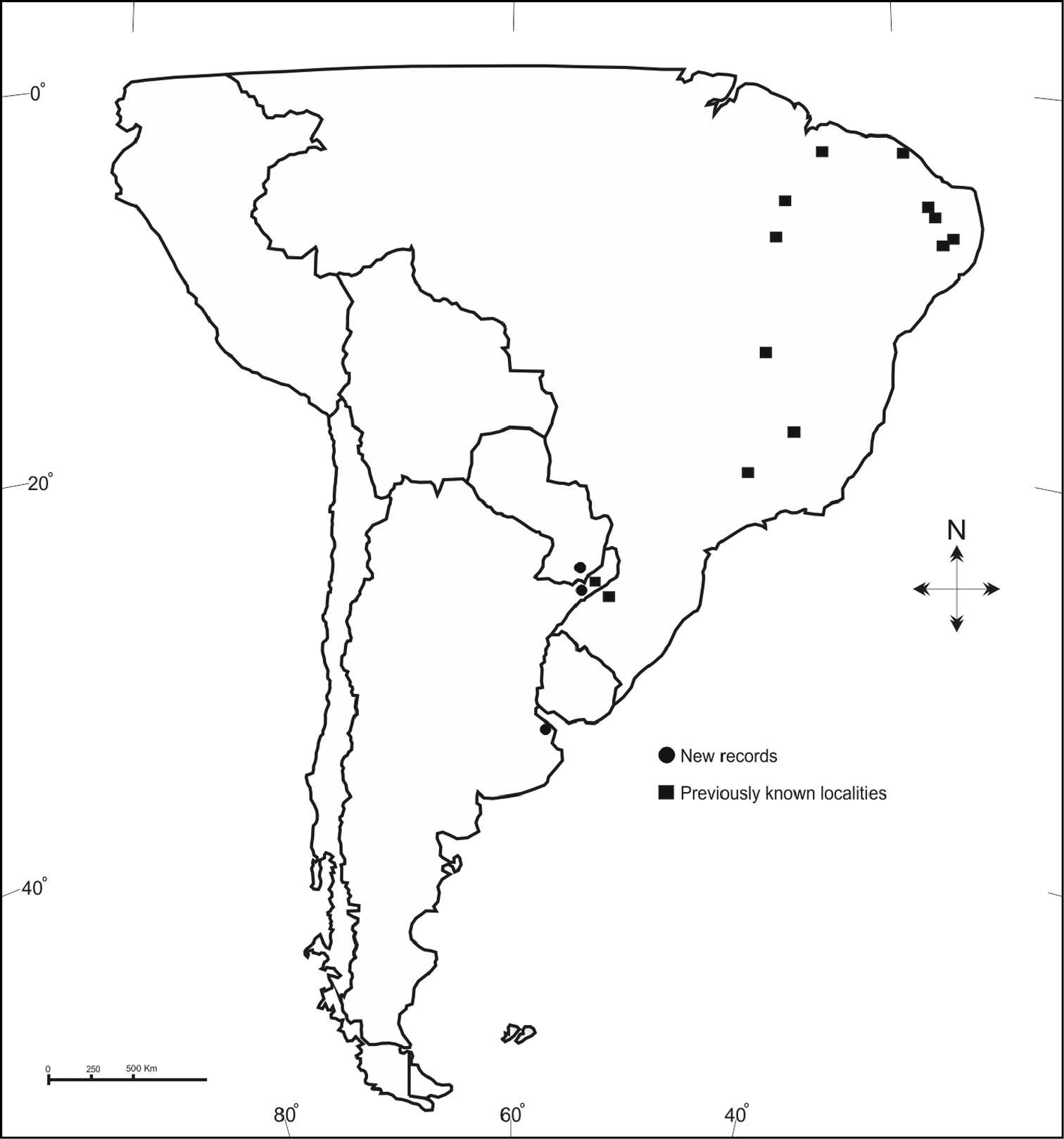
Figure 7.
Distribution records of Lithurgus huberi Ducke, including the new records discussed herein.
Acknowledgements
We are grateful to David Notton for his constant and valuable support and who kindly arranged the loan of the syntypes of Lithurgus atratus and to two anonymous reviewers for comments and suggestions that improved this note. This project was supported in part by US National Science Foundation grant DBI-1057366 (to M.S.E) and by the Department of Biological Sciences, Southwestern Oklahoma State University. This is a contribution of the Division of Entomology, University of Kansas Natural History Museum.
ReferencesCamillo E, Garófalo CA, Campos MJO, Serrano JC (1983) Preliminary notes on the biology of
Lithurgus huberi (Hymenoptera, Megachilidae). Revista Basileira de Biologia 43(2): 151–156.
Camillo E, Garófalo CA, Serrano JC (1994) Nesting activities and nest reuse of
Lithurgus huberi (Hymenoptera, Megachilidae). Revista Basileira de Biologia 54 (2): 183-194.
Ducke A (1907) Contribution a la connaissance de la faune hyménoptérologique du Nord-Est du Brésil. Revue d’Entomologie 26: 73-96.
Ducke A (1908) Contribution à la connaissance de la faune hyménoptérologique du Nord-Est du Brésil. Revue d’Entomologie 27: 57-87.
Ducke A (1910) Explorações botânicas e entomológicas no Estado do Ceará. Revista Trimestral do Instituto do Ceará 24: 3-61.
Gonzalez VH, Griswold T, Praz CJ, Danforth B (2012) Phylogeny of the bee family Megachilidae (Hymenoptera: Apoidea) based on adult morphology. Systematic Entomology 37(2): 261–286. doi:
10.1111/j.1365-3113.2012.00620.x
IABIN (2012) Inter-American Biodiversity Information Network. Avalible at:
http://www.IABIN.net [accessed on 19 September 2012]
Michener CD (1965) A classification of the bees of the Australian and South Pacific regions. Bulletin of the American Museum of Natural History 130: 1-362.
Michener CD (2007) The Bees of the World[2
nd Edition]. Johns Hopkins University Press, Baltimore, MD, 953 pp.
Moure JS, Melo GAR (2007) Lithurgini Newman, 1834. In: Moure JS, Urban D, Melo GAR (Eds) Catalogue of Bees (Hymenoptera, Apoidea) in the Neotropical Region. Sociedade Brasileira de Entomologia, Curitiba, Brazil, 914–917. [total volume pages xiv+1058 pp.] [updated at URL:
http://www.moure.cria.org.br/catalogue (last accessed 20 September 2012)]
Pick RA, Schlindwein C (2011) Pollen partitioning of three species of Convolvulaceae among oligolectic bees in the Caatinga of Brazil. Plant Systematics and Evolution 293(1/4): 147–159. doi:
10.1007/s00606-011-0432-4
Roig-Alsina A, Michener CD (1993) Studies of the phylogeny and classification of long-tongued bees (Hymenoptera: Apoidea). University of Kansas Science Bulletin 55 (4): 124-162.
Roig-Alsina A (2006)
Hylaeus punctatus (Brullé) (Colletidae), a Palaearctic bee long established in South America. Journal of Hymenoptera Research 15 (2): 286-289.
Snelling RR (1983) The North American species of the bee genus
Lithurge (Hymenoptera: Megachilidae). Contributions in Science, Natural History Museum of Los Angeles County 343: 1-11.
Wittmann D, Hoffman M (1990) Bees of Rio Grande do Sul, southern Brazil (Insecta, Hymenoptera, Apoidea). Iheringia, Série Zoologia 70: 17-43.