(C) 2013 Kyohei Watanabe. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
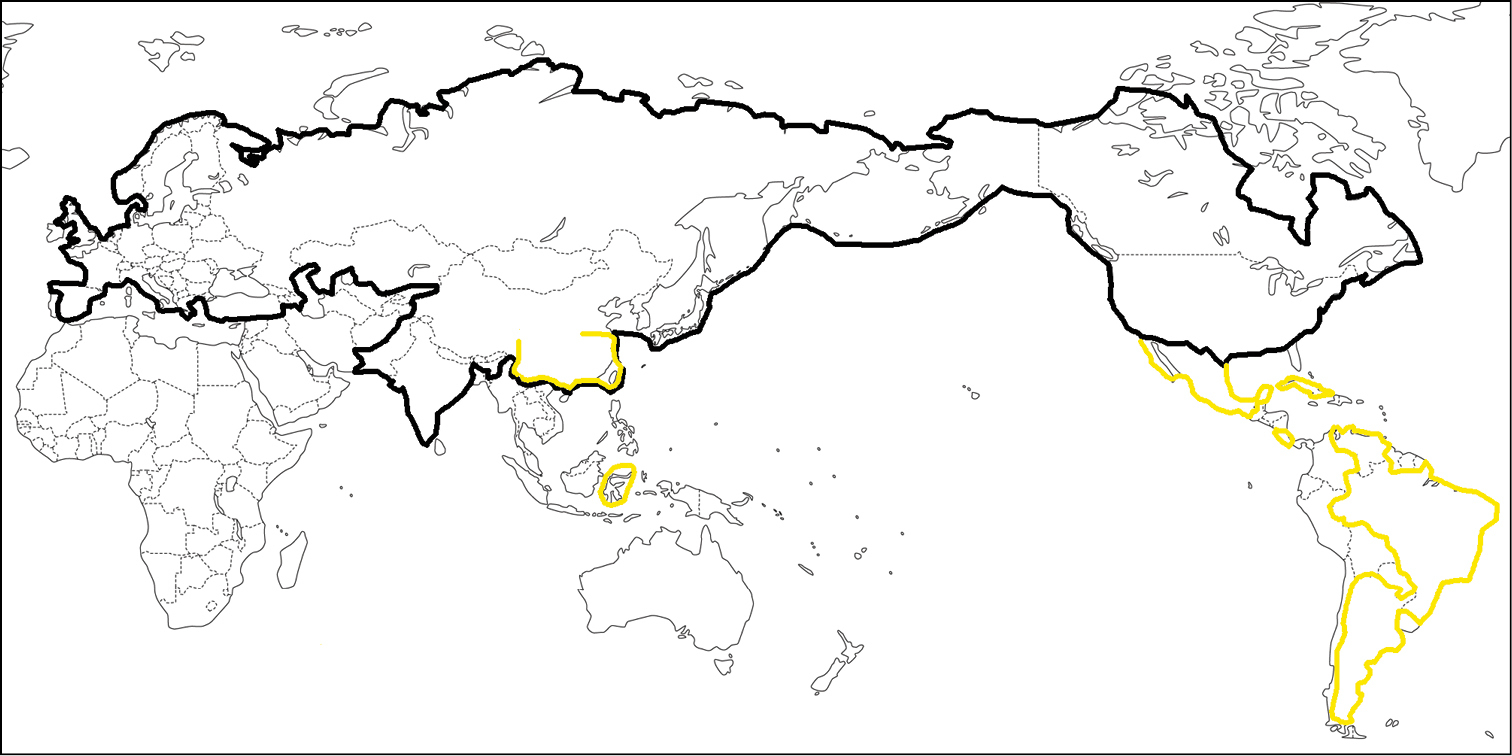
Apechthis cantika sp. n. (Ichneumonidae: Pimplinae) is described from the island of Sulawesi Indonesia. This is the first known representative of the genus in Indonesia and also the southernmost record of Apechthis in the Oriental region extending the known distribution far southwards. Discovery of a second yellow Apechthis in the Oriental region following Apechthis taiwana Uchida suggests that the genus suddenly changes its body colour entirely from black to yellow towards the equator independently in both the Oriental and Neotropical regions. The holotype of Apechthis taiwana apparently closely related to Apechthis cantika sp. n. is also redescribed for comparison.
Body colour, convergence, hamuli, Oriental region, parasitoid wasp, taxonomy, yellow body
The genus Apechthis is a small taxon of the tribe Pimplini, subfamily Pimplinae, containing 16 described species from the Holarctic, Oriental and Neotropical regions (
Body colouration, an important set of characters in morphological identification, also strongly relates to biological functions, such as thermoregulation, crypsis or warning mimicry in bumblebees (
Body colour within Apechthis shows two patterns, i.e. predominantly black or yellow, seemingly with some trend in its distribution. Most of the yellow species are found in the Neotropical region (
We had an opportunity to collect on Sulawesi Island, Indonesia in 2011, resulting in acquisition of an undescribed yellow Apechthis species (Figs 1–4), caught in a Malaise trap at c. 2000m elevation on Mt. Lompobattang (alt. 2, 870m), southern Sulawesi. In this paper, we record this genus from Sulawesi Island for the first time, describe this new, yellow species as Apechthis cantika sp. n., and discuss the distribution of body colour trends and asymmetric numbers of hamuli in wings found in two Oriental species. The holotype of Apechthis taiwana, apparently closely related to Apechthis cantika sp. n., is also redescribed for comparison.
Within range of vision, Mt. Lompobattang has been cultivated up to c. 1700m, coexisting with natural forest, and has a meadow from c. 1800 to 1900m. There is a huge cloud forest starting from c. 1900m with several creeks but seemingly fewer insects flying. We deployed Malaise traps at four altitudes each, such as 700, 1400, 1700 and 2000m, for about 40 days. Unfortunately, the traps at 700 and 1700m were stolen or destroyed by local people. The trap at 2000m, the only trap which caught Apechthis cantika sp. n., had been deployed inside the cloud forest alongside the creek.
Observation and drawings were made by stereomicroscope (Nikon SMZ800) and light microscope (Nikon eclipse 50i). Male terminalia were treated with 10% KOH at about 20ºC for 24 hours then washed in distilled water and observed in 70% ethanol. Digital images were edited using Adobe Photoshop® CS3.
Morphological terminology mainly follows
The specimens examined in this study are deposited in the following collections: the Ehime University Museum, Matsuyama, Japan (EUM), the Kanagawa Prefectural Museum of Natural History (KPMNH), the Indonesian Institute of Sciences, Bogor, Indonesia (LIPI), and the Hokkaido University Museum, Sapporo, Japan (SEHU).
http://species-id.net/wiki/Apechthis
Morphologically, this genus can easily be distinguished from other genera of Pimplini by the yellow face of the male, the mandibular teeth of equal length, the strongly notched inner margin of the eye, the non-divided clypeus (Fig. 5), and the hook-like (apically down-curved) ovipositor tip (Fig. 16) (
urn:lsid:zoobank.org:act:E9D47938-E070-474D-9D13-A5421F10BAAF
http://species-id.net/wiki/Apechthis_cantika
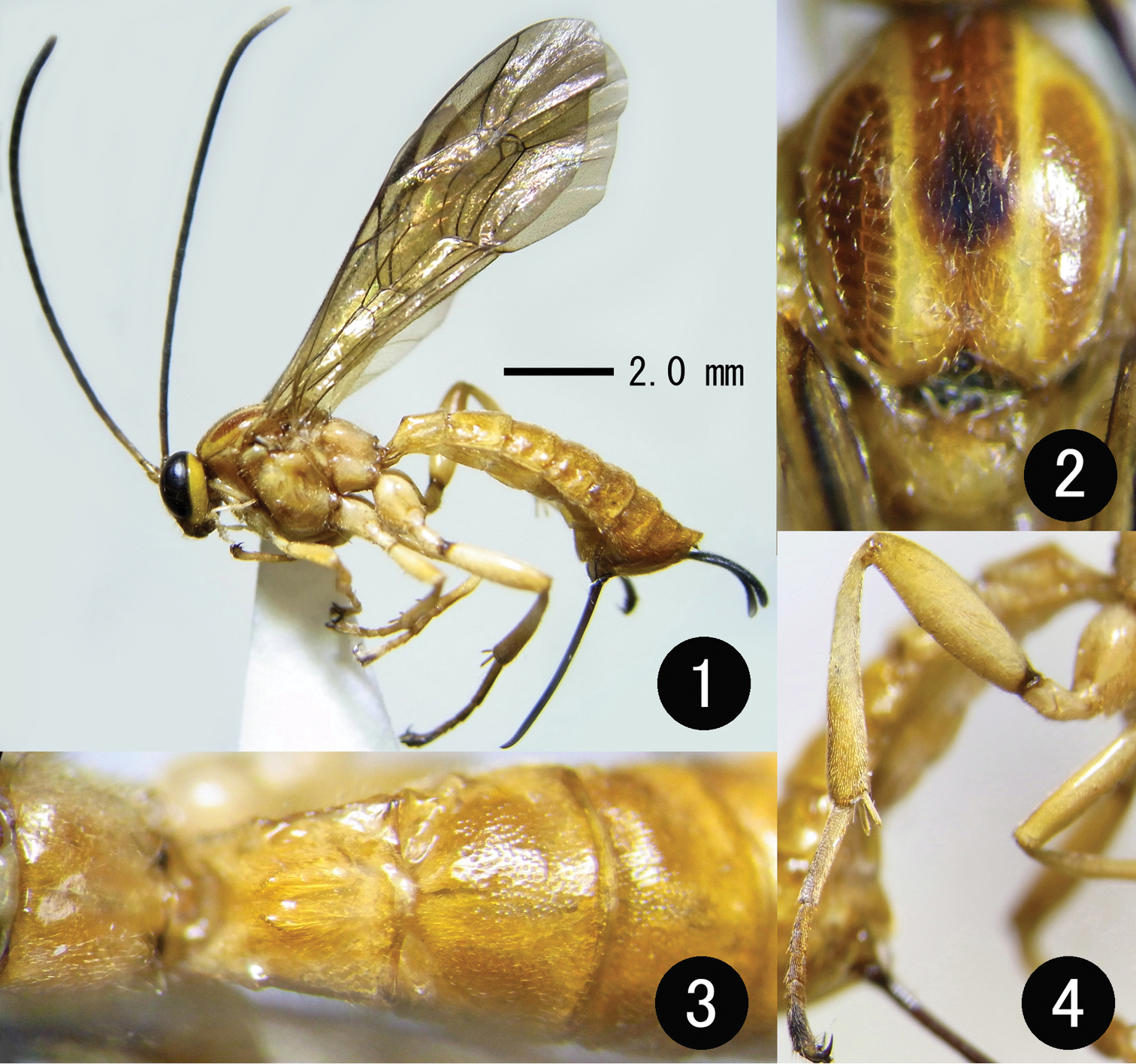
Figs 1–16, 18–22Propodeum largely smooth medially, without median longitudinal carinae (Figs 3, 14); metasomal tergite I with pair of strongly convex keels and strongly angulate in lateral view (Figs 1, 15); all tarsal claws with basal tooth (Figs 10–12); male flagellum with longitudinal ridge on flagellomere VI to basal half of IX (Fig. 6); metasoma entirely yellow (Figs 1, 3).
Description. Female: body 10.0–12.0 (HT: 12.0) mm long, fore wing 10.0–12.0 (HT: 12.0) mm long. Head polished, punctate, 0.5 times as long as wide in dorsal view; clypeus 0.6 times as long as wide, flat, smooth, finely punctate along apical margin and supraclypeal suture; face 0.7 times as long as wide, finely punctate, medially with weak longitudinal convexity (Fig. 5); frons smooth, weakly concave above antennal sockets; malar space 0.2 times as long as basal width of mandible; basal portion of mandible flat; vertex and gena minutely and finely punctate; OOL/OD 0.7; POL/OD 1.0; antenna with 25–27 (HT: 27) flagellomeres, terminal segment with columnar projections (Figs 7, 8); flagellomere I 1.3 times as long as flagellomere II.
Mesosoma polished, sparsely punctate; pronotum laterally largely smooth; mesoscutum finely, densely punctate; anterior half of mesopleuron strongly convex; upper end of epicnemial carina reaching lower apex of pronotum (Fig. 9); episternal scrobe large; lower division of metapleuron largely smooth excluding along pleural carina; propodeum smooth medially, sparsely punctate laterally, with pleural carina and posterior part of lateral longitudinal carina (Figs 3, 14, 15); lateromedian longitudinal carina present only as basal tubercle (Figs 3, 14); other carinae absent.
Legs: claws of all legs with basal tooth (Figs 10–12); hind femur 3.5 times as long as deep.
Wings: fore wing with vein Cu-a distad to vein Rs+M by 0.4–0.5 (HT: 0.5) times length of vein Cu-a, with rectangular areolet receiving vein 2m-cu near apical 1/3 (Fig. 13); hind wing with distal abscissa of vein Cu1 much closer to vein 1A than to vein M, basal abscissa of vein Cu1 0.5 times length of vein cu-a; 9 (left) or 11 (right) distal hamuli.
Metasoma polished, punctate; tergite I 1.1–1.2 (HT: 1.1) times as long as maximum width, smooth, with pair of strong median convexities (Figs 1, 3, 14, 15), convexity nearly angulate in lateral view (Figs 1, 15); tergite II 0.8 times as long as maximum width, anterior 1/5, gastrocoeli and posterior margin smooth (Figs 3, 14); tergites III to VII punctate excluding posterior margin; lower valve of ovipositor with 13 teeth (Fig. 16); ovipositor sheath 0.8 times as long as hind tibia.
Colour (Figs 1-4). Body yellow, except for: apex of mandible, dorsal surfaces of scape and pedicel, flagellomere I and II, small median spot on mesoscutum, pair of small spots on posterior margin of propodeum, apices of claws (including teeth) of legs, base of hind femur, wing veins including stigma, ovipositor, ovipositor sheath excluding apex black; posterior part of vertex, scuto-scutellar groove, apex of hind tibia, hind tarsus excluding base of first segment blackish-brown; above antennal socket on frons, ocellar area, three longitudinal stripes on mesoscutum (these stripes sometimes darkened), small areas near epicnemial carina, epistomal scrobe, subalar prominence brown; wings yellowish-hyaline.
Male: similar to female, except following characters: body 8.0–9.0 mm long, fore wing 8.0–8.5 mm long; clypeus 0.5–0.6 times as long as wide; face slightly wider, 0.7–0.8 times as long as wide; OOL/OD 1.0; POL/OD 0.7; flagellum with longitudinal ridge on flagellomere VI to basal half of IX (Fig. 6); tergite I 1.2–1.3 times as long as maximum width; tergite II 1.0 times as long as maximum width; and 8 (left) or 9 (right) distal hamuli.
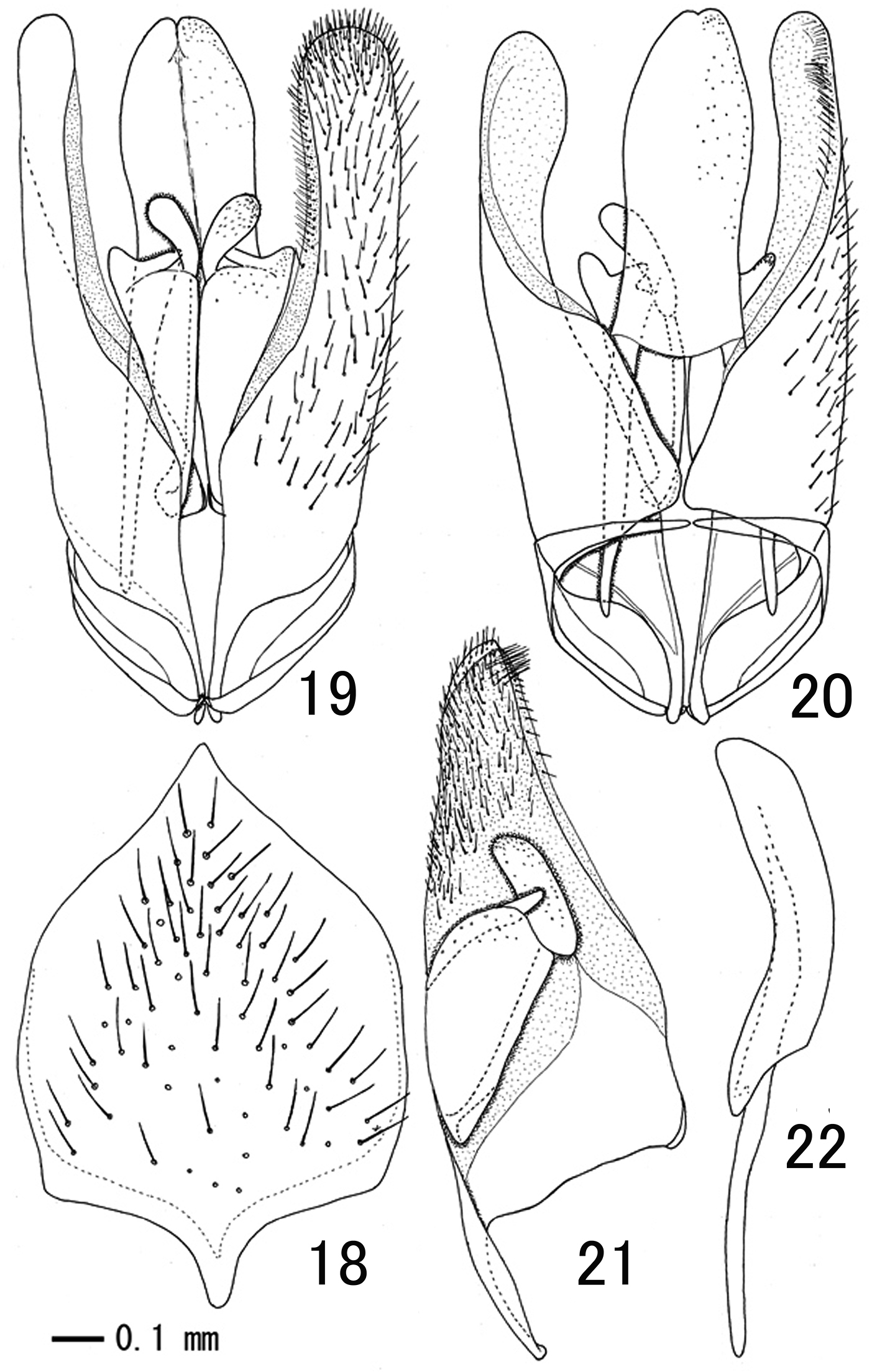
Male terminalia: subgenital plate elongate, with acute apex, apico-lateral area without setae (Fig. 18); paramere with membranous inner surface, apex covered with dense setae and ventral margin with very dense, long setae near apex (Figs 19-21); apical margin of paramere sharp, apex not projecting beyond apex of aedeagus (Figs 19, 20); aedeagus weakly widened apically, with basal apodeme slightly shorter than penis valve (Fig. 22).
Colourationsimilar to female but brown areas somewhat darker.
Holotype of Apechthis cantika sp. n. 1 habitus, lateral view 2 mesonotum, dorsal view 3 T1-T2, dorsal view 4 right hind leg, lateral view.
Holotypes (female) of Apechthis cantika sp. n. (5, 7–16) and Apechthis taiwana Uchida (17), paratype (male) of Apechthis cantika sp. n. (6) 5 Head, frontal view 6 left flagellum, lateral view 7, 8 apex of flagellum, dorsal (7) and lateral (8) views 9, 17 anterior half of mesopleuron, lateral view 10–12 left fore- (10), mid- (11) and hind (12) tarsal claw, lateral view 13 fore wing areolet 14, 15 propodeum and metasomal tergites I and II, dorsal (14) and lateral (15) views; 16 apex of ovipositor, lateral view.
Male genitalia and subgenital plate of Apechthis cantika sp. n. (paratype) 18 subgenital plate, ventral view; 19, 20 genital capsule, ventral (19) and dorsal (20) views 21 left paramere, inner aspect 22 aedeagus, lateral view.
Holotype: Female, Mt. Lompobatang, Gunung, South Sulawesi Prov., INDONESIA, 1977m alt., 14.xii–21. i.2011, Keizo Takasuka leg. (Malaise trap, 5°23'26.74"S, 119°56'1.14"E) (LIPI). Paratypes: 1 female and 2 males, same data as holotype (1 male in LIPI; 1 female and 1 male in EUM).
Indonesia (Sulawesi).
The specific name is derived from the Indonesian word “cantik”, meaning “beautiful”.
This species can easily be distinguished from most other Apechthis species by its yellow body without dark areas on the metasomal tergites (Figs 1-4), and from all yellow Neotropical species by the keel on the first metasomal tergite being strongly angulate in lateral view (Figs 1, 15 arrow) (weaker or not angulate in the Neotropical species). Although this species morphologically much resembles Apechthis taiwana, it can be distinguished by the upper end of the epicnemial carina reaching the lower apex of the pronotum (Fig. 9) (in taiwana it extends dorsally, reaching the middle of the pronotum: Fig. 17), smoother metasomal tergites I and II (Figs 3, 14) (taiwana relatively densely punctate: Fig. 24), and the entirely yellow metasoma (Figs 1, 3) (taiwana with conspicuous black or brown areas: Figs 23, 24). This species is also the southernmost representative of Apechthis in the Oriental region.
Holotype of Apechthis taiwana Uchida. 23 habitus, dorso-lateral view 24 T1-T3, dorso-lateral view 25 labels.
Although the columnar projections on the terminal antennal segment of females (Figs 7, 8) was pointed out as a synapomorphy in Pimplini (
Asymmetric numbers of hamuli in “left” and “right” wings were observed in two Oriental species, Apechthis cantika sp. n. (L<R: 2 females and 2 males, asymmetry at a rate of 100%) with stable numbers of hamuli and Apechthis taiwana (L>R: 1 female, holotype, asymmetry at a rate of 100%, see following description). But in two Palaearctic species collected from Japan, Apechthis capulifera (Kriechbaumer, 1887) (19 females and 9 males deposited in KPMNH, asymmetry at a rate of ca. 47%) and Apechthis rufata (Gmelin, 1790) (18 females and 12 males deposited in KPMNH, asymmetry at a rate of ca. 47%), although asymmetric hamuli numbers are also found in approximately half of specimens, both the side of asymmetry and numbers of hamuli are unstable, not supporting the apparently stable asymmetry trend observed in the Oriental species, but based on small numbers of specimens.
http://species-id.net/wiki/Apechthis_taiwana
Figs 17, 23, 24Although this species also has a yellow body and is found in the Oriental region, China and Taiwan (
Female: body 10.5 mm long, fore wing 10.0 mm long. Head polished, punctate, 0.6 times as long as wide in dorsal view; clypeus 0.6 times as long as wide, flat excluding weakly concave apical half, smooth, finely punctate along apical margin and supraclypeal suture; face 0.7 times as long as wide, finely punctate, medially with weak longitudinal convexity; frons smooth, weakly concave above antennal sockets; malar space 0.2 times as long as basal width of mandible; basal portion of mandible flat; vertex and gena minutely and finely punctate; OOL/OD 0.6; POL/OD 1.1; antenna with 25 flagellomeres, terminal segment with columnar projections; flagellomere I 1.3 times as long as flagellomere II.
Mesosoma polished, sparsely punctate; pronotum laterally largely smooth; mesoscutum finely, densely punctate; anterior half of mesopleuron strongly convex; upper end of epicnemial carina reaching half height of pronotum (Fig. 17); episternal scrobe large; lower division of metapleuron largely smooth except along pleural carina; propodeum smooth medially, densely punctate laterally, with pleural carina and posterior part of lateral longitudinal carina; lateromedian longitudinal carina present only as basal tubercle; other carinae absent.
Legs: claws of all legs with basal teeth; hind femur 3.0 times as long as deep.
Wings: fore wing with Cu-a distad Rs+M by 0.4 times length of Cu-a, with rectangular areolet receiving 2m-cu near apical 1/3; hind wing with distal abscissa of Cu1 much closer to 1A than to M, basal abscissa of Cu1 0.3 times as long as cu-a; 11 (left) or 10 (right) distal hamuli.
Metasoma polished, densely punctate (Fig. 24); tergite I 1.1 times as long as maximum width, with pair of strong median convexities, convexity nearly angulate in lateral view; tergite II 0.8 times as long as maximum width, gastrocoeli and posterior margin smooth; posterior margin of tergites III to VII smooth; lower valve of ovipositor with at least 10 teeth (apex concealed by dirt); ovipositor sheath 0.9 times as long as hind tibia.
Colour (Figs 23, 24). Yellow; apex of mandible, small median spot on posterior margin of mesoscutum, scuto-scutellar groove, small spot on mesopleuron near subtegular ridge and episternal scrobe, anterior margin of propodeum, pair of small spots on posterior margin of propodeum, apices of claws (including teeth) of legs, wing veins excluding stigma, anterior band of metasomal tergite I, pair of anterior spots on tergites II to VI and ovipositor sheath (apex concealed by dirt) black; antenna, ocellar triangle, three longitudinal stripes on mesoscutum, submetapleural carina, base of mid femur, apex of hind coxa, base and apex of hind femur, base and apical half of hind tibia, stigma excluding black margins, tarsal claws, ovipositor brown; each black area more or less framed by brown area; wings yellowish-hyaline.
Specimen examined. Holotype (Fig. 25): Female, “Formosa, Kikuchi” (label 1), “Yakanron, 18/II. 1927” (label 2), “A. taiwana n.” (label 3), “Type Matsumura” (label 4), apical parts of right antenna and left hind tarsus, and left fore wing lost (SEHU).
In terms of body colour, Apechthis species show two conspicuous patterns, black or yellow (Figs 1–4). Although the black species usually have conspicuous yellow mark(s) on the mesoscutum and scutellum, there are no noticeable intermediate patterns between basically yellow and black in any species. Black species are mainly Holarctic and Himalayan (
Distribution of predominantly black and predominantly yellow species of Apechthis at the country scale, based on
The authors would like to express their cordial thanks to Prof Kaoru Maeto (Kobe University) for his continuous guidance, to Dr Gavin Broad (Natural History Museum, London) for his critical reading of an early manuscript, to Dr Ilari Sääksjärvi (Zoological Museum, University of Turku, Turku) for his kind advice, and to Dr Masahiro Ohara (SEHU) for loan of the holotype of Apechthis taiwana. This study was conducted under research permission from RISTEK (No. 0005/FRP/SM/I/09), and supported by the Heiwa Nakajima Foundation for KT.