(C) 2013 Donald L. J. Quicke. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of Cystomastacoides van Achterberg, Cystomastacoides asotaphaga Quicke sp. n., is described and illustrated based on a series of specimens reared from caterpillars of the erebid moth Asota plana Walker from Papua New Guinea. Two other new species without biological data are also described, Cystomastacoides nicolepeelerae Quicke & Butcher sp. n. also from Papua New Guinea, and Cystomastacoides kiddo Quicke & Butcher sp. n. from Thailand. A key is provided to the four known species. The new species extend the known range of the genus considerably, it having previously been known only from a single species from mainland China (Yunnan), and additionally provides the first host record for the genus. Other related genera are parasitoids of Sphingidae, Lymantriidae and Crambidae.
Cytochrome oxidase I, DNA barcoding, Lepidoptera, hosts, Rogadini, Erebidae
Cystomastacoides van Achterberg, 1997, was described based on a single specimen of a new species, Cystomastacoides coxalis van Achterberg from Yunnan, China, collected as an adult (in
Here we describe three new species of Cystomastacoides, two from Papua New Guinea, the other from Thailand. Specimens of the first of these were reared from caterpillars of the erebid moth Asota plana Walker, 1854 (Lepidoptera, Erebidae), as part of an extensive herbivore and parasitoid rearing programme (
Terminology follows
Abbreviations: BMNH (The Natural History Museum, London); QSBG (Queen Sirikit Botanic Gardens, Chiang Mai, Thailand); USNM (United States National Museum, Washington D.C.).
| 1 | 1st metasomal tergite less than 2.0 × longer than posteriorly wide (Fig. 1); mesoscutum medioposteriorly broadly depressed, without distinct oval pit (Fig. 2); propodeum anteriorly largely shiny with deep punctures (Fig. 3); pterostigma bicolorous, basally nearly black, apically pale yellow (Fig. 5); wing with dark grey transverse band at level of parastigma (Fig. 5); 3rd segment of maxillary palp [female] cylindrical, approximately 7 × longer than wide; length of fore wing less than 10mm [8mm] | Cystomastacoides kiddo sp. n. |
| – | 1st metasomal tergite more than 2.0 × longer than posteriorly wide (Fig. 9); mesoscutum medioposteriorly with deep, narrow oval pit (Fig. 11); propodeum anteriorly largely rugose (Fig. 12); pterostigma unicolorous black or yellow brown (Fig. 9) or dark anteriorly and yellowish posteriorly; wing membrane entirely hyaline or apically infuscate; 3rd segment of maxillary palp [female] swollen, approximately 3.5 × longer than wide (Fig. 10); length of fore wing more than 10mm [11.5–12mm] | 2 |
| 2 | Occipital carina strongly wavy (Figs 10, 11); pterostigma entirely black; wing membrane entirely hyaline | Cystomastacoides asotaphaga sp. n. |
| – | Occipital carina more or less evenly rounded; wing membrane at least weakly infuscate apically | 3 |
| 3 | Hind wing vein 2-SC+R distinctly transverse, reclivous (Fig. 21); pterostigma largely dark brown, but distinctly black medioanteriorly (Fig. 16); 3rd segment of maxillary palp 5 × longer than maximally wide (Fig. 19) | Cystomastacoides nicolepeelerae sp. n. |
| – | Hind wing vein 2-SC+R quadrate; pterostigma and venation yellow brown; 3rd segment of maxillary palp 3.1 × longer than maximally wide | Cystomastacoides coxalis van Achterberg |
urn:lsid:zoobank.org:act:B08D1758-3EFE-4FE4-AC76-E79A7D0C1106
http://species-id.net/wiki/Cystomastacoides_kiddo
Figs 1–8Holotype female, Thailand, Phetchabun Province, Thung Salaeng Luang NP, Kaeng Wang Nam Yen, 29.xi–6.xii.2006, 16°37.531'N, 100°53.745'E, Malaise trap (collection code T1165; voucher BCLDQ00783; Genbank JX034704; BOLD: ASQSQ064_09) (QSBG)
Length of body 9 mm, of fore wing 8 mm and of antenna 12 mm.
Head. Antenna with 59 flagellomeres. Terminal flagellomere acuminate. Median flagellomeres approximately 3 × longer than wide. 3rd segment of maxillary palp and 2nd segment of labial palp not swollen, rather elongate, 3rd segment 6 × longer than maximally deep. Face rather protruding and with well developed transverse striation. Frons with strong oblique striation reaching to midlongitudinal groove. Distance between posterior ocelli: transverse diameter of posterior ocellus: shortest distance between posterior ocellus and eye = 1:3.2:2.5. Vertex with strong groove running from stemmaticum to occipital carina. Occipital carina complete, strongly lamelliform laterally, weak mediodorsally.
Mesosoma. Notauli very deep, crenulate posteriorly, meeting before scutellar sulcus. Mesoscutum without midposterior pit. Scutellar sulcus wide with single carina medially. Mesopleuron largely smooth and shiny with some small punctures, precoxal sulcus long, weakly impressed, largely smooth. Median area of metanotum with complete midlongitudinal ridge. Propodeum largely shiny with deep punctures, with lateral carinae distinct posteriorly and protruding.
Fore wing. Vein 2-CU1 5.0 × longer than 1-CU1. Vein 3-SR 3.0 × longer than r. Vein SR1 1.45 × longer than 3-SR.
Hind wing. Vein M+CU approximately as long as 1-M. Vein 2-SC+R quadrate (interstitial). Vein m-cu absent.
Metasoma. 1st metasomal tergite 1.65 × longer than posteriorly wide; posteriorly with well-developed shoulders. 2nd metasoma 1.15 × longer than 3rd tergite, and 1.1 × wider than long. Metasomal tergites 1-3 with strong midlongitudinal carina. Tergites 1-6 densely deeply punctate with punctures forming irregular longitudinal rows separated by striae. Hypopygium weakly curved ventrally.
Coloration. Antennae largely black. Body and legs largely pale brown yellow, apex of hind tibia and hind tarsus and stemmaticum black. Wing venation largely brown yellow, basal 0.6 of pterostigma and veins at same level dark brown; membrane uniformly hyaline.
Figures 1–4. Cystomastacoides kiddo sp. n. holotype, Cell^D® light photomicrographs. 1 Habitus 2 head and anterior mesosoma, dorsal view 3 propodeum, dorsal view 4 metasomal tergites 2-6, dorsal view.
Figures 5–8. Cystomastacoides kiddo sp. n. holotype, Cell^D® light photomicrographs. 5 Fore wing 6 metasoma, lateral view 7 apex of hind tibia showing spurs 8 hind coxa showing dorsal tooth (arrowed).
Named after the character Beatrice Kiddo in the Quentin Tarantino ‘Kill Bill’ films because of the deadly biology to the host.
urn:lsid:zoobank.org:act:88ADE279-A5EC-436D-A9DC-DB8935839809
http://species-id.net/wiki/Cystomastacoides_asotaphaga
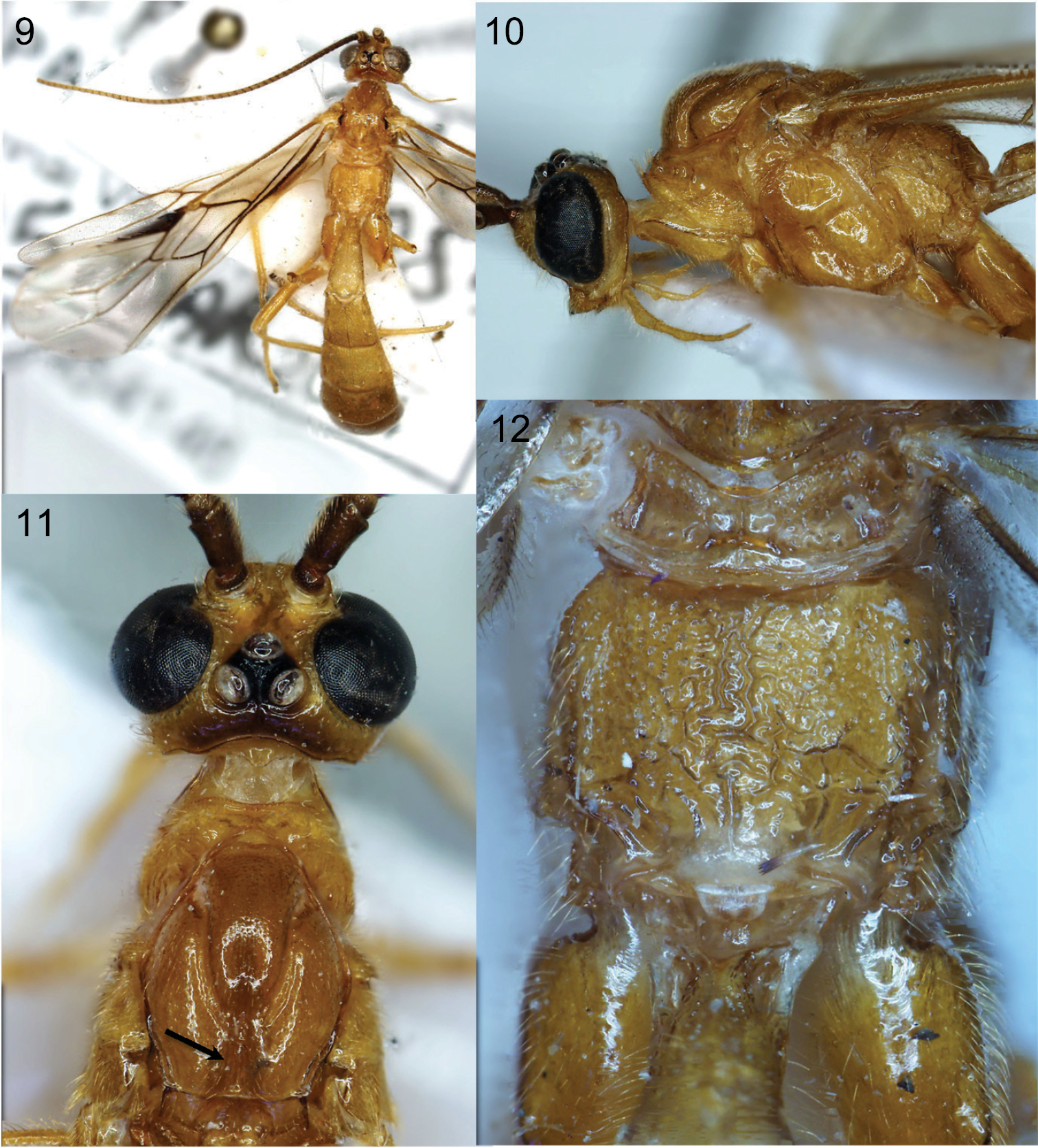
Figs 9–15Holotype female, Papua New Guinea, East Sepik Province, Yapsiei, 5.iii.04, 4°37.695'S, 141°05.839'E, 100m, PAR, CATJ085, reared ex caterpillar of Asota plana (Walker) (Erebidae, formerly Noctuidae: Erebini) on Ficus pachyrrhachis K. Schum. & Laut. (Moraceae). Voucher USNM ENT 00454146; Genbank JF271304; BOLD: ASQSP054-08) (USNM).
Paratypes, 2 females and 1 male, same data as holotype: (USNM ENT 00454262, Genbank JX034706, BOLD ASQSP050-08; USNM ENT 00454362, Genbank JX034703, BOLD ASQSP051-08; USNM ENT 00454359, Genbank JX034707, BOLD ASQSP052-08) (USNM, BMNH, USNM respectively).
Length of body 12 mm, of fore wing 16 mm and of antenna 14 mm.
Head. Antenna with 74 flagellomeres. Terminal flagellomere acuminate. Median flagellomeres approximately twice as long laterally as wide. 3rd segment of maxillary palp and 2nd segment of labial palp with rather large flattened, triangular flange, 3rd segment 3.0 × longer than maximally wide. Face with rather strongly protruding midlongitudinal area, largely densely punctate with punctures largely confluent giving rise to transverse rugulose appearance. Frons weakly obliquely striate on lateral half only. Distance between posterior ocelli: transverse diameter of posterior ocellus: shortest distance between posterior ocellus and eye = 1:1.8:0.6. Vertex with strong groove running from stemmaticum to occipital carina. Occipital carina complete, distinctly wavy, produced to a point mediodorsally strongly lamelliform laterally, weak mediodorsally.
Mesosoma. Notauli very deep, almost smooth though with weak crenulation posteriorly, almost meeting just anterior to deep midposterior pit on mesoscutum. Scutellar sulcus wide with single carina medially. Mesopleuron largely smooth and shiny with moderately dense small punctures, precoxal sulcus long, moderately impressed, rather narrow, with weak rugulose sculpture. Propodeum strongly and extensively rugose, with lateral carinae distinct posteriorly and protruding.
Fore wing. Vein 2-CU1 3.6 × longer than 1-CU1. Vein 3-SR 2.1 × longer than r. Vein SR1 1.3 × longer than 3-SR.
Hind wing. Vein M+CU 1.8 × length of 1-M. Vein 2-SC+R distinctly transverse, reclivous. Vein m-cu absent.
Metasoma. 1st metasomal tergite 2.2 × longer than posteriorly wide. Metasomal tergites 1, 2 and 3 with strong midlongitudinal carina. Tergites 1-4 densely deeply punctate with punctures forming longitudinal rows separated by weak longitudinal to sublongitudinal striae. Tergite 4 densely finely punctured with little trace of striation. Tergite 5 largely smooth and shiny with few punctures. Hypopygium strongly curved ventrally.
Coloration. Antennae blackish basally becoming yellow brown gradually from near middle to apex. Body and legs largely brown yellow, posterior metasomal tergites and hypopygium somewhat darker, stemmaticum black. Wing venation and pterostigma largely black brown, fore wing veins C+SC+R and 1-R1 brown yellow, membrane uniformly hyaline.
Figures 9–12. Cystomastacoides asotaphaga sp. n. holotype, Cell^D® light photomicrographs. 9 Habitus 10 head and mesosoma, lateral view 11 head and anterior mesosoma, dorsal view, arrow indicating medioposterior pit 12 propodeum.
Figures 13–15. Cystomastacoides asotaphaga sp. n. holotype, Cell^D® light photomicrographs. 13 Hind wing 14 hind coxae 15 metasomal tergites 2-6, dorsal view.
Name means feeding on Asota.
urn:lsid:zoobank.org:act:21D6462F-AB7A-42BC-9F26-433D27EF27CD
http://species-id.net/wiki/Cystomastacoides_nicolepeelerae
Figs 16–24Holotype female, Papua New Guinea, Kokoda I, ix–x.1933, 200ft, L. E. Cheesman (museum code B.M. 1933-427) (BMNH)
Paratype [probably] female, Papua New Guinea, Oro Province, Kokoda, x.1933, 1200ft, L. E. Cheesman (museum code B.M. 1933-321) (BMNH)
Length of body 13 mm, and of fore wing 11.2 mm.
Head. Antenna incomplete, with at least 44 flagellomeres. Median flagellomeres approximately 2.5 × as long laterally as wide. 3rd segment of maxillary palp 5 × longer than maximally wide with weak expansion; 2nd segment of labial palp with rather large flattened, triangular flange. Face with moderately protruding midlongitudinal area, largely densely punctate with punctures largely confluent giving rise to transverse rugulose appearance. Frons with two oblique striae between antennal socket and eye, extending slightly behind antennal socket, otherwise smooth. Distance between posterior ocelli: transverse diameter of posterior ocellus: shortest distance between posterior ocellus and eye = 1.25:4.5:1.0. Vertex with weak groove running from stemmaticum to occipital carina. Occipital carina complete, more or less smoothly rounded.
Mesosoma. Notauli very deep, punctate anteriorly, crenulated posteriorly, almost meeting just anterior to deep midposterior pit on mesoscutum. Scutellar sulcus wide with single carina medially. Mesopleuron largely smooth and shiny with some small punctures at the bases of setae, precoxal sulcus long, moderately impressed, rather narrow, almost completely smooth. Propodeum strongly and extensively rugose, with lateral carinae distinct posteriorly and protruding.
Fore wing. Vein 2-CU1 4.1 × longer than 1-CU1. Vein 3-SR 2.3 × longer than r. Vein SR1 1.1 × longer than 3-SR.
Hind wing. Vein M+CU 1.4 × length of 1-M. Vein 2-SC+R distinctly transverse, reclivous. Vein m-cu absent.
Metasoma. 1st metasomal tergite 2.3 × longer than posteriorly wide. Metasomal tergites 1, 2 and 3 with strong midlongitudinal carina. Tergites 1-4 densely deeply punctate with punctures forming longitudinal rows separated by weak longitudinal to sublongitudinal striae. Tergite 4 densely finely punctured with little trace of striation. Tergite 5 largely smooth and shiny with few punctures. Hypopygium strongly curved ventrally.
Coloration. Antennae blackish basally becoming yellow brown gradually from near middle to apex. Body and legs largely brown yellow, posterior metasomal tergites somewhat darker, hypopygium and sides of tergite 6 brown-black, stemmaticum black. Pterostigma largely dark brown but with anteromedially black. Fore wing veins C+SC+R and basal half of M+CU, paler brown yellow, wing membrane largely hyaline but distinctly apically infuscate.
Figures 16–20. Cystomastacoides nicolepeelerae sp. n. Cell^D® light photomicrographs. 16 Habitus 17 face 18 head, dorsal view 19 head and mesosoma, lateral view 20 mesoscutum, dorsal view.
Figures 21–24. Cystomastacoides nicolepeelerae sp. n. Cell^D® light photomicrographs. 21 propodeum, posterodorsal view 22 detail of hind wing vein 2-SC+R 23 metasomal tergite 1, dorsal view 24 metasomal tergites 2-3, dorsal view.
Named in honour of Nicole Peeler, one of DQ’s favourite authors.
The host species, Asota plana, is a widespread species from the Oriental tropics to New Guinea, and from lowland to montane habitats, and is known to feed on multiple species of Ficus (Moraceae) (
Other related genera are parasitoids of Arctiidae (Cystomastax:
On the basis of the barcoding COI gene fragment alone, the above two species of Cystomastacoides (the only two for which molecular data are available) do not come out as a monophyletic group when analysed together with all other available sequences for members of the Colastomion group using either maximum likelihood or maximum parsimony (Quicke et al. in prep.). Instead both associate with one subgroup of Colastomion species, including Colastomion wanang Quicke and some other undescribed/unidentified species, members of the genus Colastomion also appearing as three rather widely separated groups intermixed with other Colastomion group genera, despite all having been reared from Crambidae. To determine whether either of these genera is monophyletic will probably require combined analyses of data from multiple genes as well as denser taxonomic sampling (see
We are grateful to Scott Miller for his identification of and comments on the host. We thank the New Guinea Binatang Research Center parataxonomists, village assistants, Lauren Helgen, and Legi Sam for technical assistance, Mike Sharkey for allowing study of the Thai specimen collected as part of the TIGER project (see http://sharkeylab.org/tiger ), Scott E. Miller for host identification and and George Weiblen for plant identification, and Kees van Achterberg for supplying additional drawings of the holotype of Cystomastacoides coxalis. This paper stems from a rearing campaign led by Vojtech Novotny, George Weiblen, Scott E. Miller and Yves Basset and supported by the US National Science Foundation (DEB 0841885, 0816749, 0515678), Grant Agency of the Czech Republic (206/08/H044, 206/09/0115 and P505/10/0673) and the Czech Ministry of Education (LC06073, ME9082, and MSM6007665801). Laboratory reagents and Barcode of Life Datasystem (BOLD; http://www.boldsystems.org ) infrastructure were funded by Genome Canada through the Ontario Genomics Institute. The Animal Systematic Research Unit and Integrated Ecology Lab, Department of Biology, Faculty of Science, Chulalongkorn University kindly allowed use of their Cell^D® imaging facility.