(C) 2013 Michael J. Sharkey. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Sharkey MJ, Stoelb SAC (2013) Revision of Agathacrista new genus (Hymenoptera, Braconidae, Agathidinae, Agathidini). Journal of Hymenoptera Research 33: 99–112. doi: 10.3897/JHR.33.4373
Based on a cladistic analysis, a new genus of Agathidini, Agathacrista Sharkey, is proposed and its phylogenetic position hypothesized. Two previously described (Agathacrista cancellata, Agathacrista depressifera) and three new species (Agathacrista sailomi, Agathacrista winloni, Agathacrista krataeii) are included. The distribution of Agathacrista is limited to the Oriental region and southern portion ofthe eastern Palearctic.
Insecta, identification key, taxonomy, systematics
Agathacrista, as proposed here, includes two previously described species, both of which were included in the paraphyletic concepts of Bassus s.l. and Therophilus s.l. This paper is part of a series that investigates these non-monophyletic taxa, while describing taxa from Thailand, Costa Rica, or more inclusive areas of the world.
Morphological terms: The length of the first metasomal tergite is measured from the apex of the tendon emanating from the propodeum to the posterior border of the tergite. Other terms are from
Museum acronyms
HIC Hymenoptera Institute Collection, University of Kentucky, Department of Entomology, Lexington, Kentucky, USA.
QSBG Queen Sirikit Botanic Gardens, Chiang Mai, Thailand.
TARI Taiwan Agricultural Research Institute, Entomology Collection, Taichung, Taiwan.
Species description format: Descriptions are of the holotype; variation is given in parentheses. In the species descriptions color is not extensively described because the images serve this purpose; however color characters that are variable or of diagnostic significance are detailed.
Species delimitation: The five species from Thailand treated here are very similar morphologically and are represented by only seven specimens. The seven specimens do not differ more than might be expected within one species of agathidine. In part, we relied on 28S sequence data to separate species. We did not employ any particular cut-off point in differences in 28S but rather calibrated the amount of sequence difference using species that exhibit significant morphological and host preference differences. This calibration comes from a large dataset of about 1, 000 agathidine specimens for which we have 28S data. The three species of Agathacrista for which we have 28S sequences were identified according to this criterion. Morphological characters also played an important role in species delimitation. We examined ten specimens of Agathacrista cancellata (Enderlein, 1920), all from Taiwan. Body color varies considerably in this species, but wing color and the sculpture and dimensions of the first metasomal median tergite are uniform, suggesting that these are good species indicators within the genus. Therefore, these characters played an important role in our species concepts. Agathacrista depressifera (van Achterberg & Long, 2010) displays considerable variation in wing color-pattern. Despite the fact that we have some evidence to suggest that this species concept may include more than one species, our evidence is weak and not convincing enough to suggest changes. This revision of Agathacrista is very preliminary; many more specimens will be needed to fully understand species limits and species diversity.
Generic concepts: This is the fourth of a series of papers (
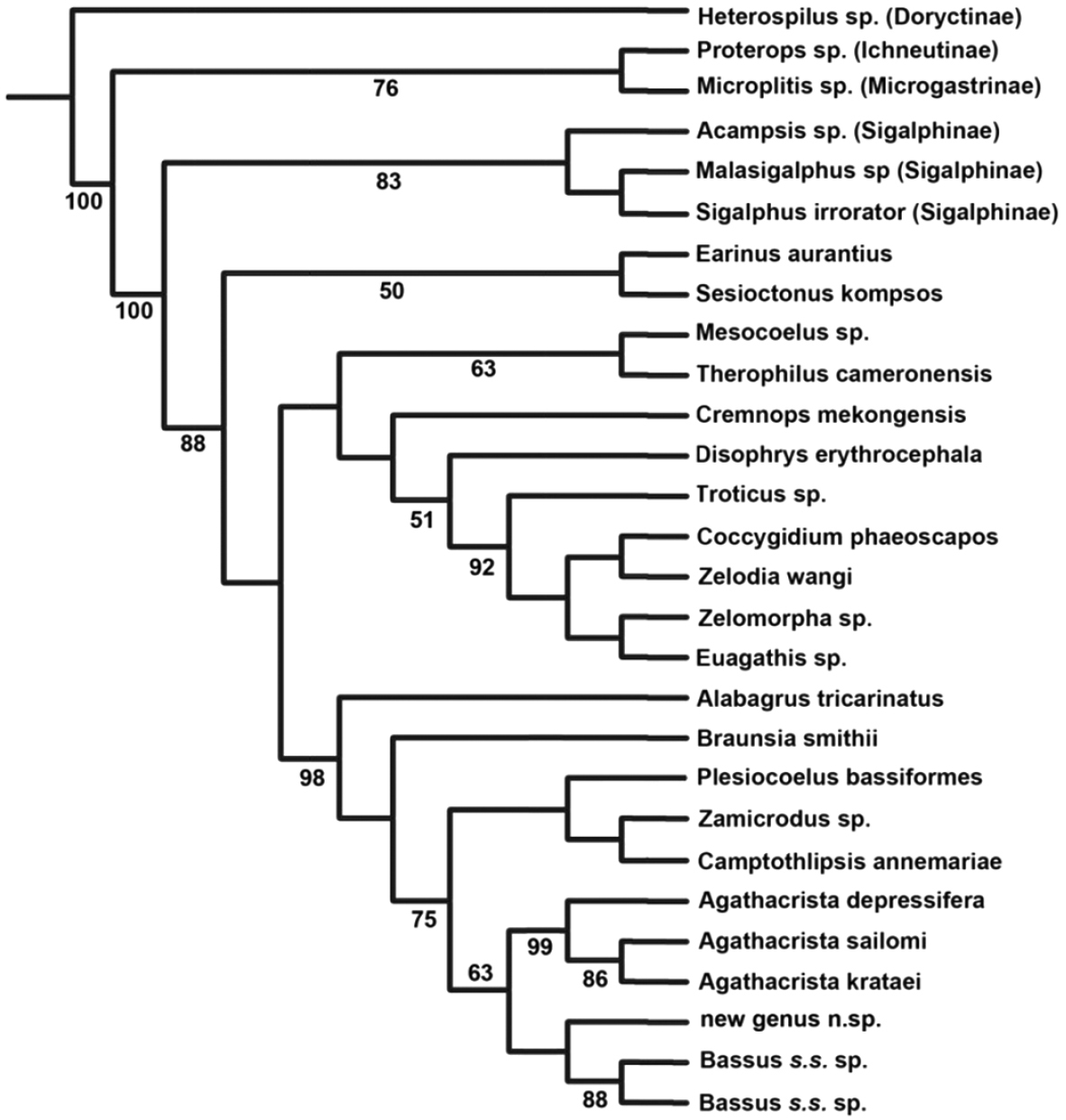
Identifiability: Agathacrista is a good example of the utility of this criterion. Agathacrista, Bassus s.s. and a new genus to be described in a forthcoming paper, form a monophyletic group (Fig. 2). The only morphological synapomorphy to aid in the recognition of the group is the presence of small spines or pegs on the fore tibia; convergent in some members of Earinus and many other non-agathidines. If there was an easily observed character it might be best to expand the concept of Bassus to include Agathacrista and the new genus. Conversely, all three genera as here proposed have distinguishing characteristics. Bassus s.s. has long, simple claws that lack a basal lobe; Agathacrista has a crest between the antennae and the undescribed new genus has a distinct longitudinal carina on the hind trochanter, a character formerly thought to be restricted to some members of the Cremnoptini and Disophrini.
Biological information content: Typically the more that is known about organisms the more finely split the higher ranks are. This criterion has little application to any agathidines since we know so little about their natural history. Undoubtedly the morphological autapomorphies of the three genera referred to above will be shown to relate to unique behaviours.
Stability: This is somewhat tied to most other criteria and Bassus s.l. is a good example of an unstable concept. Over the years it has been included in the genus Agathis s.l. and numerous genera like Camptothlipsis and Therophilus have been included, or not. This instability has made information retrieval on any particular species rather cumbersome, as the number of different combinations they have been referred to is a minimum of three, i.e., Microdus, Bassus, Agathis, the foremost being a junior objective synonym of Bassus. Thus, generic concepts should be accompanied by solid phylogenetic evidence when such is available.
Phylogenetic evidence: This is directly related to stability. If new, but weak evidence suggests the non-monophyly of a well-recognized genus, it is usually best to wait for more data before making formal changes. In the case presented here, there is strong molecular phylogenetic evidence suggesting that the broad concept of Bassus (Bassus s.l.) is polyphyletic and there is significant morphological and molecular evidence for the monophyly of Agathacrista.
Specimen collection: As part of the inventory of Thai insects, 3 Malaise traps at each of 30 different localities throughout Thailand were operated from 2007-2010, comprising approximately 90 trap-years. The specimens dealt with here are primarily from these traps.
Phylogenetic methods: Regions D2-D3 of 28S rDNA (roughly 560 base pairs) were sequenced using the following primers: 28SD2hymF 5’ - AGAGAGAGTTCAAGAGTACGTG - 3’ and 28SD3hymR 5’ - TAGTTCACCATCTTTCGGGTC - 3’. Sequences were edited using Geneious Pro v4.7.5 (
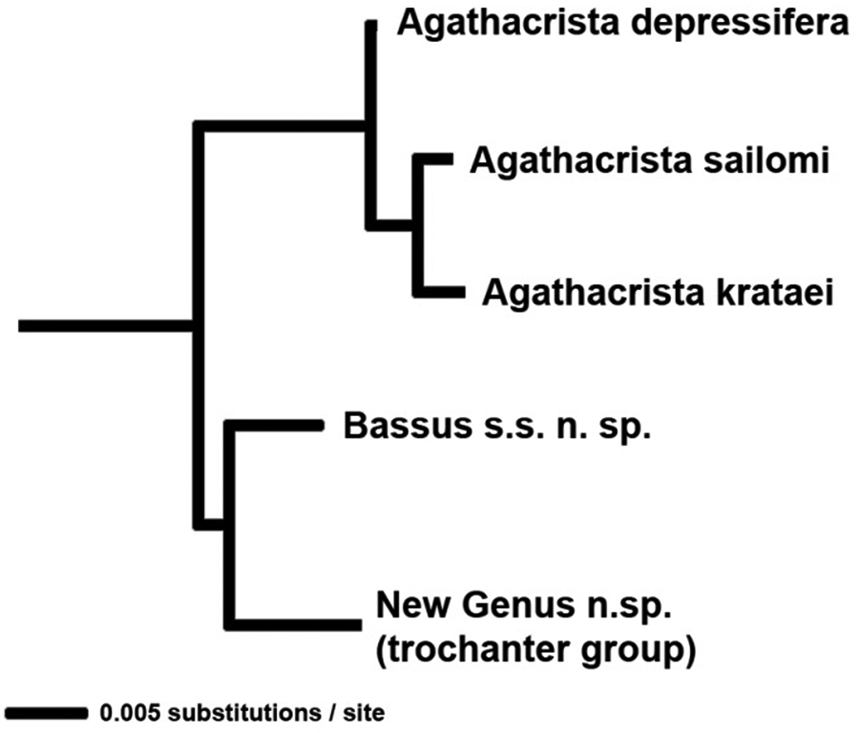
The NJ analysis (Fig. 1) was conducted with PAUP* (
NJ tree showing 28S distances between species of Agathacrista.
Maximum parsimony tree of some agathidine genera with a concentration near Agathacrista based on D2-D3 regions of 28S. Numbers below branches are bootstrap values; only values ≥50 are shown.
Morphological terms used in this revision were matched to the Hymenoptera Anatomy Ontology (HAO;
All species are treated with a diagnosis and distributional data. They are illustrated with color photos using a JVC digital camera mounted on a Leica MZ16 microscope and Automontage® stacking software. Distributional data are listed for all species and a link to a Google map is included for all species. The descriptions are of holotypes and variation is given in parentheses.
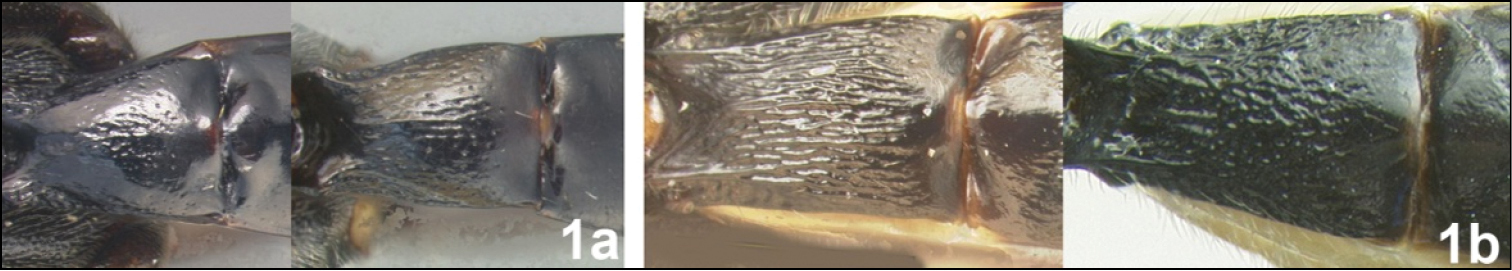
Members of all three genera have distinct pegs on the anterior surface of the fore tibia (Fig. 3a), a synapomorphy. This character state is convergent in some members of the distantly related genus Earinus (Agathidinae), some Braconinae, most Doryctinae, and has rare and scattered appearances in other braconid subfamilies. Members of Agathacrista and Bassus have similar and rather unique color patterns (see Figs 4–8). The thin, acute, interantennal crest (Fig. 3b) is an autapomorphy for Agathacrista that is found convergently though rarely in a few other agathidine genera, e.g., a few members of Therophilus.
a fore leg of Agathacrista sp. showing pegs or spines b dorsolateral head of Agathacrista sp. showing interantennal crest.
Agathacrista cancellata. a lateral habitus b lateral head and mesosoma.
Agathacrista depressifera lateral habitus.
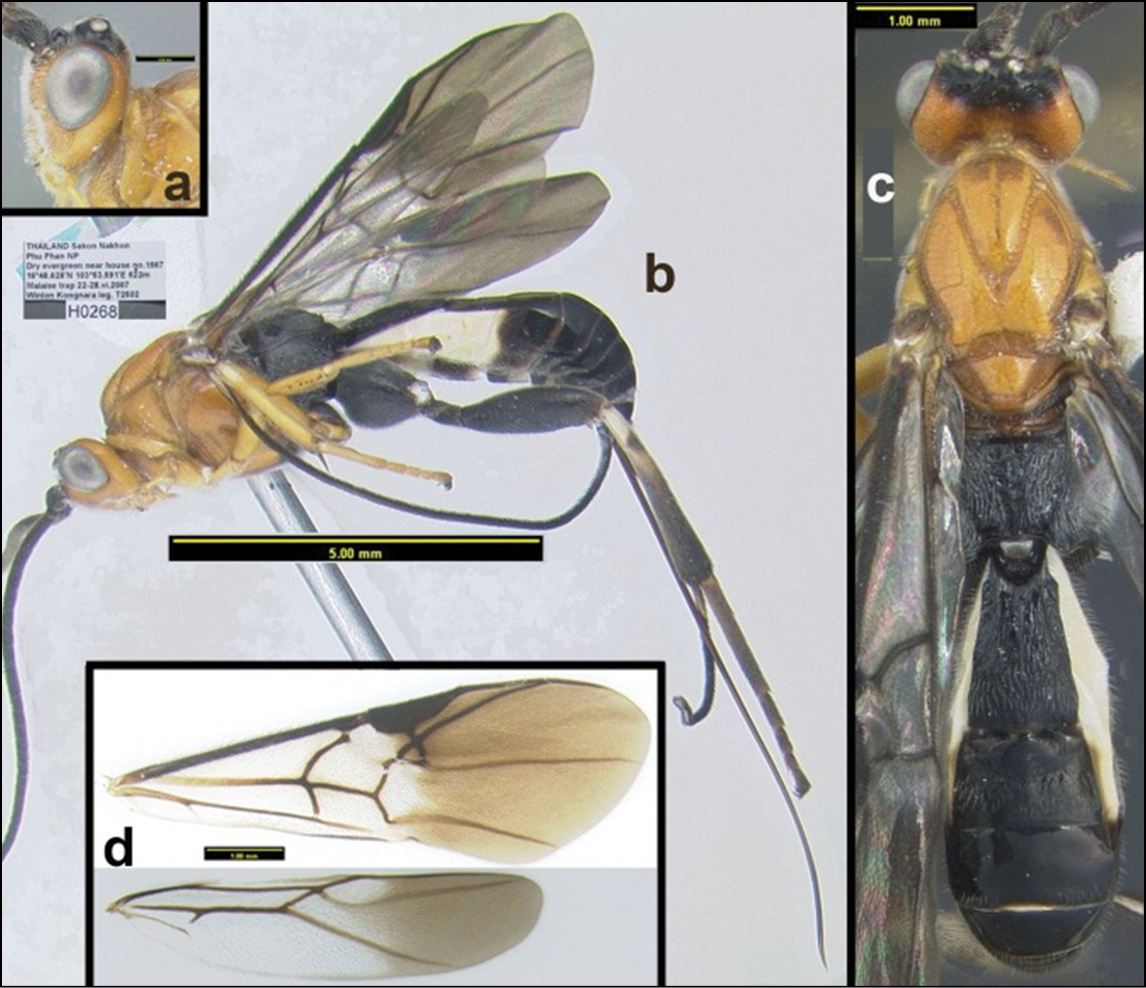
Agathacrista krataei. a lateral head b lateral habitus c dorsal habitus d wings.
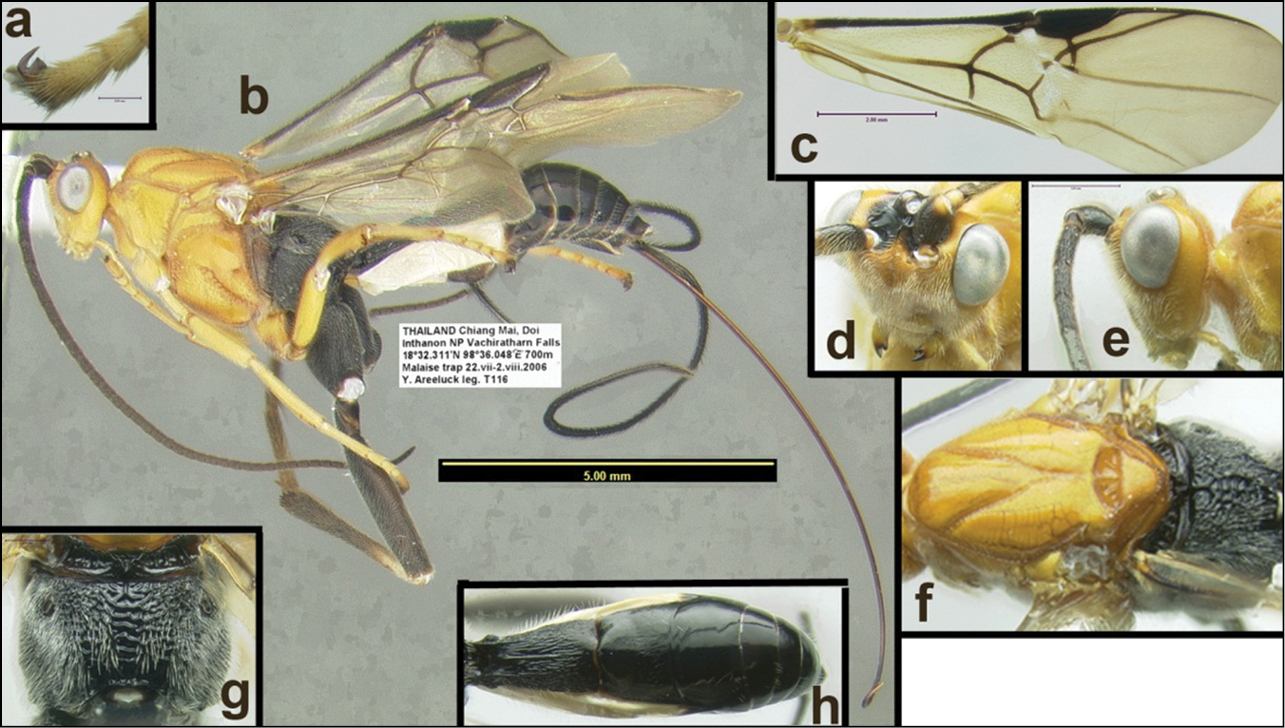
Agathacrista sailomi. a tarsal claw b lateral habitus c fore wing d anterolateral head e lateral head f dorsal mesosoma g dorsal propodeum h dorsal metasoma.
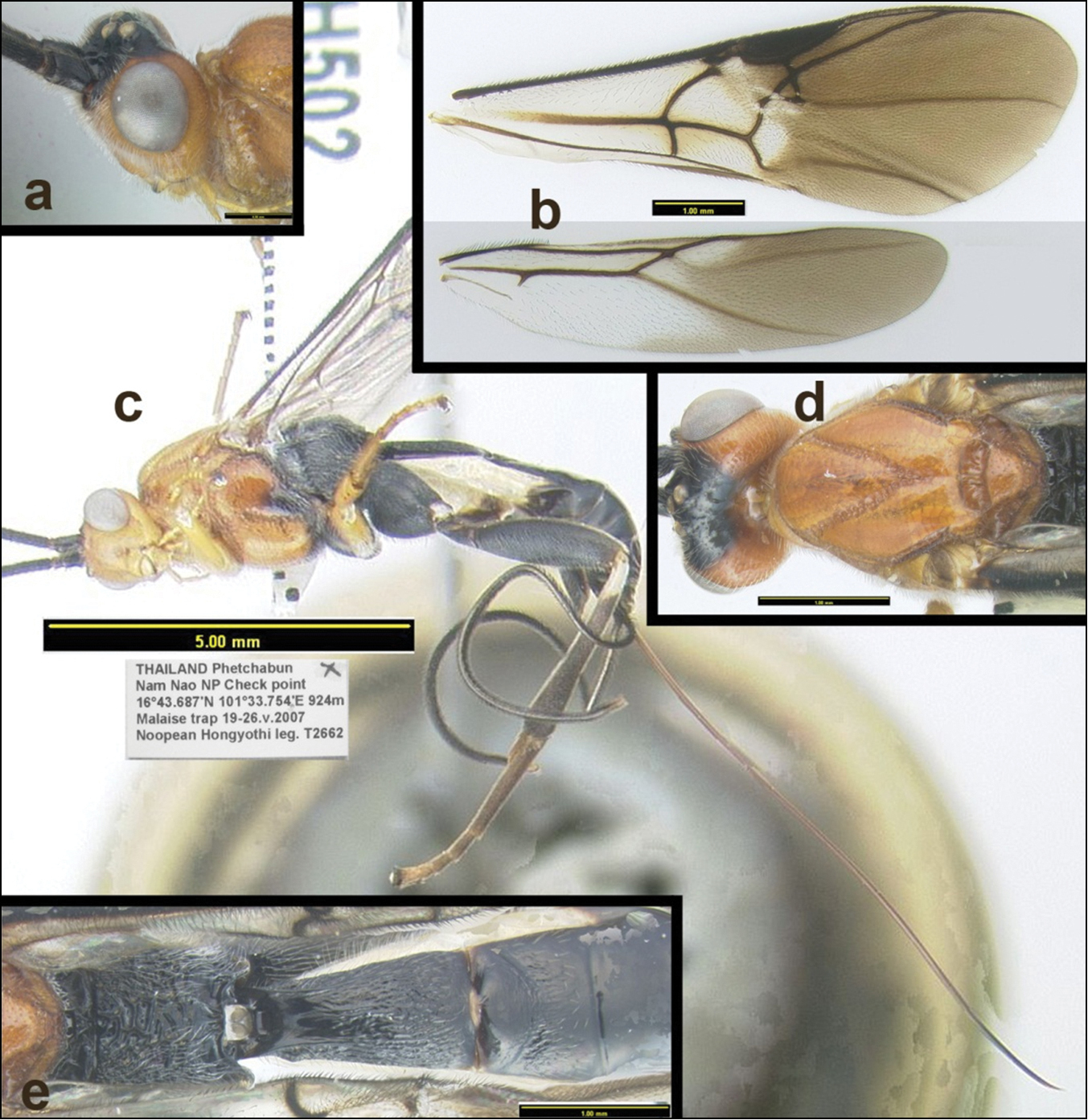
Agathacrista winloni. a lateral head b wings c lateral habitus d dorsal head and mesosoma e dorsal propodeum and metasomal median tergites 1–3.
Agathacrista winloni Sharkey, sp. n.
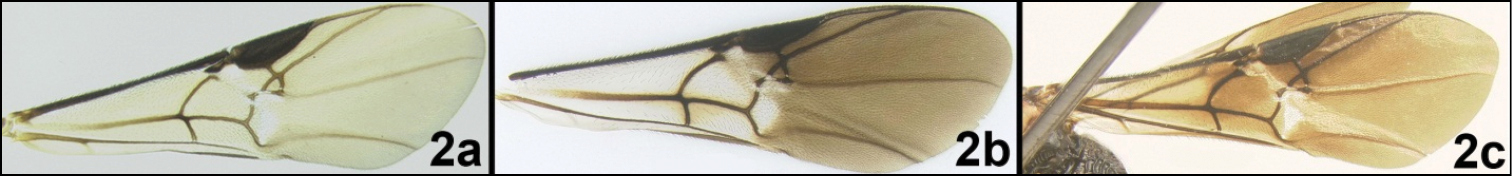
Interantennal space with longitudinal keel that is sharply declivous posteriorly (Figs 3b, 7d); ocelli elevated above surface of vertex; fore tibia with pegs (Fig. 3a); third metasomal median tergite lacking sculpture; basal lobe of tarsal claws large and right angled or slightly acute (Fig. 7a); fore wing partly or completely pigmented; hind wing vein CUb absent. Some members of a few other genera of Agathidini have a sharp keel between the antennae, but none of these have pegs on the fore tibia.
Head. Lateral carina on frons (as in members of Alabagrus) absent; interantennal space with longitudinal keel that is sharply declivous posteriorly (Figs 3b, 7d); ocelli elevated above surface of vertex; gena not extended ventroposteriorly into sharp prominence (Fig. 6a); gena lacking sharp angle posteriad of eye; labial palpus with 4 segments, third segment less than ½ as long as apical segment; apical antennomere acute but lacking nipple-like process. Mesosoma. Propleuron convex ventrally but lacking a sharp bump; notauli impressed and pitted, at least in part (Fig. 6c); posteroscutellar depression absent but sculpture usually present in this area; propodeum rugose to areolate-rugose (Fig. 7g); sclerite between hind coxal cavities and metasomal foramen complete, its ventral margin situated ventral to dorsal margin of hind coxal cavities. Legs. Fore tibia with pegs (Fig. 3a); all tarsal claws with strong basal lobe and with right-angled or slightly acute angle (Fig. 7a). Wings. (Figs 6d, 8b). Fore wing RS+M vein mostly absent; second submarginal cell triangular; fore wing 3RSb straight and strong throughout; hind wing r and r-m cross veins absent; hind wing vein CUb absent; fore wing partially or completely pigmented, with yellow or melanic color. Metasoma. First median tergite longitudinally striate, with a slightly stronger pair of lateral striae and a medial stria (Fig. 7h, 8e), rarely striae reduced and sculpture mostly weakly rugose (see couplet 1 in the identification key); second median tergite with an elevated semi-circular area separated from the remainder of the tergite by a shallow groove (Fig. 8e); second median tergite from smooth to striate, usually smooth anteriorly with a few longitudinal striae in posterior half; median tergite 3 smooth; length of ovipositor rather uniform, as long as (Fig. 5) or slightly longer than body (Fig. 8c).
Unknown
Restricted to the eastern Palearctic (Taiwan) and the Oriental region. For a distribution map, click here.
From the Greek agathis, meaning “ball of thread”, and the Latin crista, meaning “crest”. Crest is in reference to the ridge between the antennal insertions. The gender is feminine.
| 1a | Median tergite 1 mostly smooth or weakly sculptured | Agathacristacancellata |
| 1b | Median tergite 1 mostly striate or rugosostriate | 2 |
| 2a | Fore wing uniformly yellow, at most slightly darker apically | 3 |
| 2b | Fore wing clear basally, infuscate distally | 4 |
| 2c | Fore wing mostly evenly infuscate, very slightly clearer at extreme base | Agathacrista depressifera |
| 3a | Head mostly yellow, black color restricted to medial dorsal surface; larger specimens, ~ 9.6 mm | Agathacrista sailomi |
| 3b | Head mostly black, yellow color restricted to part of face and orbits of eyes; smaller specimens, ~ 6.2 mm | Agathacristadepressifera |
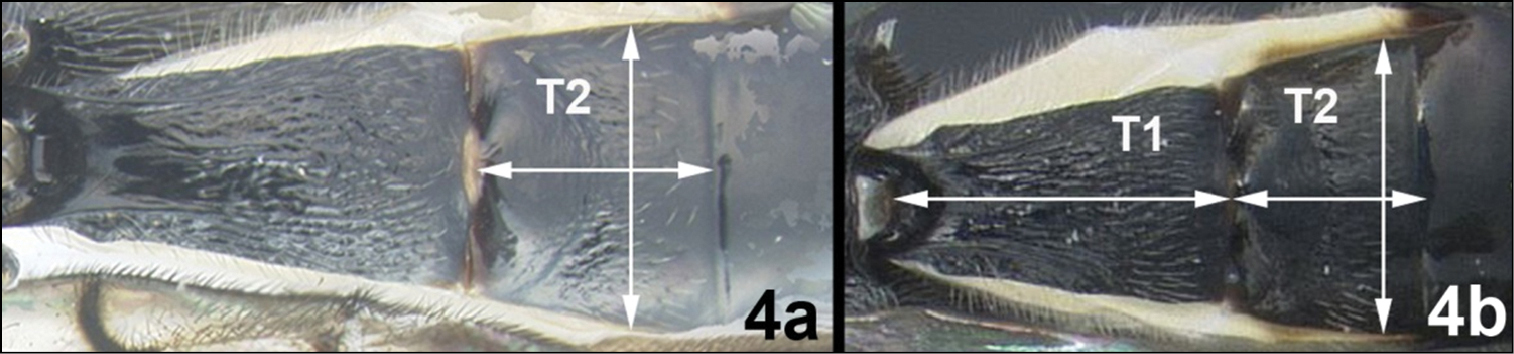
| 4a | Median tergite 1 (T1) weakly converging anteriorly; T2 quadrate and ~1.3× wider than long | Agathacrista winloni |
| 4b | Median tergite 1 strongly converging anteriorly; T2 diverging posteriorly and ~1.5× wider than long | Agathacristakrataeii |
http://species-id.net/wiki/Agathacrista_cancellata
Fig. 4This species has the northern-most distribution and appears to be restricted to Taiwan where it is the only species present. Members come in two color morphs; one with the mesosoma bicolored and the other with the mesosoma entirely melanic. Agathacrista cancellata differs from all other members of the genus by the relatively smooth surface of metasomal median tergite 1 (Fig. 1a in the identification key above).
Taiwan: Female: “Kanshirei, 20 xi 1928, col. J Sonan” (HIC). “Kanshirei, 10 xi 1926, col. J Sonan” (TARI). Female: “Taichung, Wufeng, Wanfeng, 2-7-v-1979, K.C. Chou” (HIC). Female: “Puli, Taichung. 16-19 v. 1956, K.S. Lin.” (HIC). Female: “Taipei, 15.v.1964 K.S. Lin” (TARI). Male: “Shantimen, Pingtung Hsien, 28.III.-1.IV.1981, C.C. Chen & C.C. Pang” (TARI). Female: “12.x.1952, Coll. S. C. Cheu” (TARI). Female: “Arisan, 5-VIII-1981, Col. T. Shiraki” (TARI). Female: “Chungho, 28.V.1963, Col. H.H. Tseng” (TARI). Female: “Suo-Suigen 24.VIII.1930, Col. J. Sonan” (HIC). For a distribution map, click here.
http://species-id.net/wiki/Agathacrista_depressifera
Fig. 5Length: 6.5 (7.8) mm. Antenna with 38 (42) flagellomeres. Number of spines on fore, mid, and hind tibiae 6(5), 8(11), 15(16). First median tergite 1.6 times longer than wide; second median tergite 1.2 times wider than long; median tergite 1 rugosostriate, except smooth near posterior margin, sculpture more reticulated than congeners; median tergite 2 smooth (weakly rugosostriate near midlength). Color: (melanic color of mesopleuron more extensive in the sole paratype.)
Vietnam: Female: “Dak Lak Prov. Krong Bong, Chu Yan Sin NP, 12°31.303'N, 108°28.853'E, 28.vii.2008 MT” H7991 (QSBG). Thailand: Female: “Phetchabun, Khao Kho NP, 520m, 16°52.568'N, 101°08.104'E, 12–19.x.2006 Sintong leg. T807” H002 (HIC). For a distribution map, click here.
Genbank Accession KC556782 (H002).
http://zoobank.org/FCDD9F11-0FF1-4B90-A7AB-5A75BE08A235
http://species-id.net/wiki/Agathacrista_krataei
Fig. 6Length: 8.4 mm. Antenna with 41 flagellomeres. Number of spines on fore, mid, and hind tibiae 6, 10, 13. First median tergite 1.5 times longer than wide; second median tergite 1.5 times wider than long; median tergite 1 entirely rugosostriate; median tergite 2 mostly weakly sculptured with striae, smooth posterolaterally.
Named after Kratae Sanok a collector for the TIGER project at Pa Hin Ngam National Park.
Holotype: Female: Thailand, “Sakon Nakhon, Phu Phan NP, Dry evergreen near house no. 1567, 16°48.628'N, 103°53.591'E, Malaise trap, vi.2007, Winlon Kongnara leg., T2502” H268 (QSBG). For a distribution map, click here.
Genbank Accession KC556781 (H268).
http://zoobank.org/61CEE178-5F5E-4302-8A10-E4AE769CAF12
http://species-id.net/wiki/Agathacrista_sailomi
Fig. 7Length: 9.6(9.3) mm. Antenna with 44 flagellomeres. Number of spines on fore, mid, and hind tibiae 4(3), 6, 6(13). First median tergite 1.6 times longer than wide; second median tergite 1.4 times wider than long; median tergite 1 rugosostriate, except smooth near posterior margin; median tergite 2 mostly smooth with a weak indication of longitudinal sculpture at midlength. Color: (Melanic color of head restricted to ocellar triangle in the sole paratype.)
Named after Sailom Tongbunchai a collector for the TIGER project at Phuphan National Park.
Holotype: Female: Thailand: “Chiang Mai, Doi Inthanon NP, Vachiratharn Falls, 18°32.311'N, 98°36.048'E, 700m Malaise trap, 6–13.ix.2006 Y. Areeluck leg. T242” H013 (QSBG).
Genbank Accession KC556780 (H013).
Female: Thailand: “Chiang Mai, Doi Inthanon NP, Vachiratharn Falls, 18°32.311'N, 98°36.048'E, 700m Malaise trap, 22.vii–2-viii.2006 Y. Areeluck leg. T116” H284 (HIC). Female: “Chiang Mai, 20.ix-20.x.(19)84, D. Jackson” H5904 (QSBG). For a distribution map, click here.
http://zoobank.org/3556862D-AC1D-428F-818D-B8CA315212D1
http://species-id.net/wiki/Agathacrista_winloni
Fig. 8Length: 8.4 (9.8) mm. Antenna with 43(45) flagellomeres. Number of spines on fore, mid, and hind tibiae 6, 8, 12(15). First median tergite 1.8 times longer than wide; second median tergite 1.2 times wider than long; median tergite 1 rugosostriate, except smooth near posterior margin; median tergite 2 mostly smooth with a weak indication of longitudinal sculpture at midlength (almost entirely smooth). Color: (melanic color of mesopleuron more extensive in one paratype; mid femur melanic in basal half).
Named after Winlon Kongnaka a collector for the TIGER project at Phuphan National Park.
Holotype: Female: Thailand: “Phetchabun, Nam Nao National Park Check Point, 16°43.687'N, 101°33.754'E, 924m 19–26.v.2007 Noopean Hongyothi leg. T2662” H502 (QSBG).
Genbank Accession KC771135 (H502).
Female: Thailand: “Phetchabun, Nam Nao National Park Check Point, 16°43.687'N, 101°33.754'E, 924m 19–26.v.2007, Noopean Hongyothi leg. T2662” H501 (HIC). Female: “Phetchabun, Nam Nao NP Sam Makao forest, 16°41.067'N, 101°40.425'E, 540m, Malaise trap 4–11.ix.2006 Higgyothi & Janteab leg.” H466 (QSBG). Female: “800m, 180 km NE Bangkok Khao Yai Nat. Park 10-16.iv.1990 B.V. Brown, Moist evergreen for(est). MT.” (HIC). For a distribution map, click here.
We thank the staff at Queen Sirikit Botanic Garden in Chaing Mai for sorting the many hundreds of samples and for the Thai park staff for operating Malaise traps and other collection devices. A special thanks to Chaweewan Hutacharern for managing the Thai end of the TIGER project. Also many thanks to the curators around the world (London, Oxford, Warsaw, Leiden, Gainesville, Budapest, Beijing, Paris, Stockholm, Washington) for lending specimens and hosting MJS on visits. Special thanks to Kees van Achterberg for lending specimens and types from Vietnam. Funding was provided by NSF grants DEB-0542864 and EF-0337220.