(C) 2013 Laura C. Sarzetti. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Sarzetti LC, Genise FF, Sánchez VM, Farina JL, Molina AM (2013) Nesting behavior and ecological preferences of five Diphaglossinae species (Hymenoptera, Apoidea, Colletidae) from Argentina and Chile. Journal of Hymenoptera Research 33: 63–82. doi: 10.3897/JHR.33.5061
The nests of Cadeguala albopilosa (Spinola, 1851), Diphaglossa gayi Spinola, 1851, Ptiloglossa tarsata (Friese, 1900), Ptiloglossa matutina (Schrottky, 1904) and Zikanapis tucumana (Moure, 1945) (Colletidae, Diphaglossinae) from Argentina and Chile are described herein. They show similar features to those of other Diphaglossinae: they consist of a main tunnel, cells disposed radially, isolated or in pairs, and connected to the main tunnel by laterals ones. Main tunnels are mostly vertical in species nesting in soil surface but horizontal to inclined in Diphaglossa gayi, which nests in banks. Cells are vertical with curved necks. The cells of Cadeguala albopilosa show less curved necks (less than 90°), whereas in the remaining four species the cell neck is highly curved (90° or more). Cells of Ptiloglossa tarsata have a spiral earthen closure and a wad cotton-like material, whereas in Ptiloglossa matutina only had the last one. In the remaining studied species any type of closure were found. Cocoons of Cadeguala albopilosa and Ptiloglossa tarsata are coriaceous showing a closure composed of three disks. Zikanapis tucumana and possibly Ptiloglossa matutina showed dim-light foraging. The remaining species are diurnal. The climate in their nesting sites is highly diverse, ranging from 8°C to 20°C in mean annual temperature, and from 250 mm to 3000 mm in mean annual precipitation. Only Cadeguala albopilosa and, to a lesser extent, Zikanapis tucumana nested gregariously. Zikanapis tucumana and Ptiloglossa tarsata were observed visiting flowers of Solanum.
Cadeguala albopilosa, Diphaglossa gayi, Ptiloglossa tarsata, Ptiloglossa matutina, Zikanapis tucumana, Caupolicanini, Diphaglossini, nest architecture, ecological preferences
The Diphaglossinae include the largest and most robust Colletidae. They are known for being mostly crepuscular to nocturnal bees that are found in habitats ranging from deserts or near deserts to humid tropical rain forests (
The behavior and nest architecture of all Diphaglossinae is rather homogeneous and contrasts markedly with that of other colletid subfamilies (
Fossil bee cells with a curved shape attributed to Diphaglossinae have been recently recorded from the Cenozoic of Patagonia, Argentina (
In this contribution, novel nesting and biological observations are provided for three Caupolicanini species: Ptiloglossa matutina (Schrottky, 1904), Ptiloglossa tarsata (Friese, 1900), and Zikanapis tucumana Moure (1945); and two Diphaglossini species: Cadeguala albopilosa (Spinola, 1851) and Diphaglossa gayi Spinola (1851).
Excavations of nests were performed using plastic tubes to trace the tunnels downward while exposing a vertical section of the soil showing the whole structure of the nest when possible. The measures taken were: width and height of the tumulus, maximum diameter and length of the main tunnel and laterals, and number of cells. The length of the vertical part of the cell (from the bottom to the based of the neck), the maximum diameter of the cells, and the diameter of the neck were also measured. The larvae were boiled in water and maintained in alcohol 70%. Samples of soil surrounding nests were collected and carried to the laboratory for studying micromorphology. Collected bees were deposited in the entomological collection of the Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” (MACN-En), Buenos Aires (Argentina). Dr. Arturo Roig Alsina and Dr. Luis Compagnucci identified the bees. CT images were taken with Elscint SeleCT SP tomograph. 3D-reconstruction of one nest was prepared using computer program SLICER3 v.3.6.3, 2011. SEM images of cell operculum and cocoons were taken with a Philips XL30 SEM at the Museo Argentino de Ciencias Naturales.
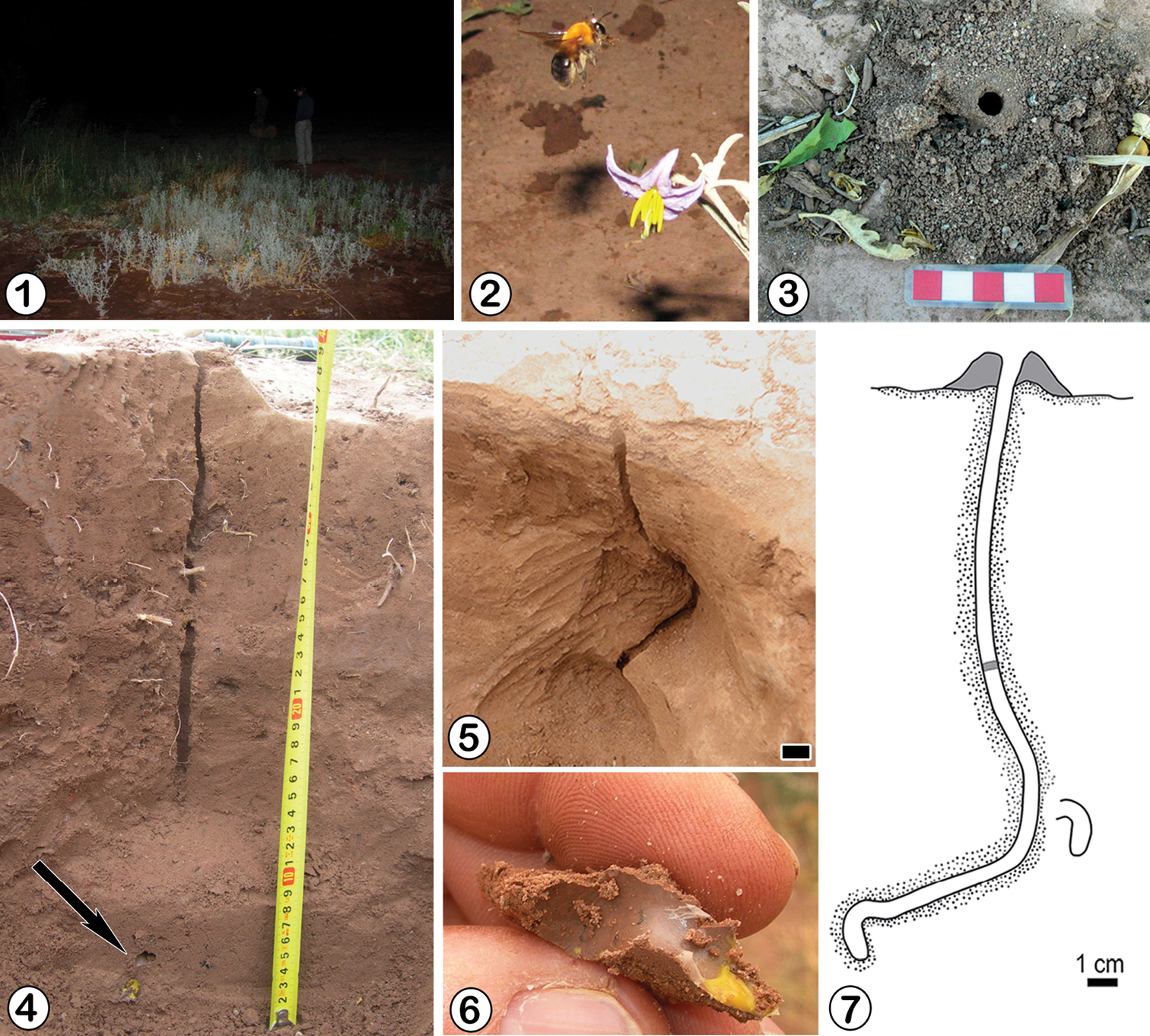
Observations on the biology and nest structure of this species were carried out during November 24th–30th, 2008 and through February 3rd–5th, 2009 at Vinchina (28°49.117'S, 68°11.433'W) and Anillaco (28°48.517'S, 66°55.867'W), both at La Rioja province, northwestern Argentina. The localities are included in xeric environments of the Larrea’s shrubland with a mean annual temperature (MAT) around 17° C and a mean annual precipitation (MAP) around 250 mm. Nest entrances were exposed in open areas and were never hidden by stones or shrubs. At Vinchina, nests formed an aggregation at the border of a formerly plowed open area frequently flooded by irrigation (Fig. 1). The ground surface was flat, compacted, and devoid of rocks. The soil was composed principally of fine sand to silt bridged by sparse clayish material. The vegetation was sparse and basically comprising plants of Solanum sp. (10 to 20 cm tall) and grasses (Fig. 1). At Anillaco the nests were found at the surroundings of the CRILAR (Centro Regional de Investigaciones Científicas y Transferencia Tecnológica de Anillaco, La Rioja). The ground surface was flat, and the soil consisted of fine sand and numerous rocks of different sizes, which hindered excavation. The vegetation was sparse, consisting of some grasses, and scarce herbaceous plants, such as Mirabilis ovata and Solanum sp., some cacti, and trees (Salix sp. and Prosopis sp.). The nests at both localities were located among plants of Solanum sp. or near them.
Zikanapis tucumana (Moure, 1945). 1 General view of the nesting site at Vinchina (La Rioja province) before sunrise 2 Female of Zikanapis tucumana during foraging activity 3 Tumulus, turret andopennest entrance 4 Main and lateral tunnel showing one cell at the end (arrow) 5 General view of nest architecture, scale line: 1 cm 6 Remains of a cell with part of the provisions. Note the curvature of neck 7 Nestarchitecture.
Zikanapis tucumana was the only species in this study that clearly demostrated dim-light, matinal foraging. At 04:30 am, still at night, the flower buds of Solanum sp. were still closed and no bee activity was observed. Females became active around 05:00 am, still at night, when the flower buds of Solanum sp. began to open (Fig. 2). After 05:20 am, with twilight, females were completely active. With sunrise, around 07:00 am, the foraging activity of the bees ceased almost completely. The activity ended definitively around 07:30 with full daylight, when the entrance of nests were closed from inside with a soil plug. The number of foraging trips per bee during these 150 minutes for the five nests observed was around 8. The females with pollen remained inside nests about 2–3 minutes before leaving again. Their foraging trips lasted about 14 to 17 minutes. During November, males of Zikanapis tucumana were also observed flying around nests 1–2 m above ground.
The entrance of nests was surrounded by a tumulus that ranged from 5 to 10 cm in diameter and 1 cm high (n: 6). Some nests also had a consolidated turret of 0.8 cm in maximum diameter and 1.7–2 cm high above the entrance (Fig. 3). The entrance, circular and 0.8 cm in diameter, was located at the center of the tumulus. The main tunnel, circular in cross section, was plugged with soil at approximately 10 cm from the entrance when the female was inside the nest. The main tunnel, 16–24 cm long (n: 9), was vertical and mostly straight at Vinchina but sinuous at Anillaco. Three nests had one cell and three other nests two cells. Cells, oriented vertically, were found at depths from 17 to 31 cm. They were disposed radially around the main tunnel, and connected with it by lateral tunnels 6-8 cm long. Lateral tunnels were subhorizontal or slightly inclined downwards and filled with soil when connected with closed cells. They ended in a raised, curved, entrance tunnel connected with the vertical portion of the cell (Figs 4, 5 and 7). Once lined and sealed, the distal part of the entrance tunnel became the curved neck of the cell. The vertical portion of the cells was 1.3–1.8 cm long and 0.9–1 cm in maximum diameter (n: 7). The neck was 0.7–0.8 cm in diameter (n: 2). The inner cell wall, including the neck, was smooth and lined with a whitish, semitransparent, cellophane-like material (Fig. 6). Two cells obtained during November from Vinchina contained eggs. The eggs, whitish, elongate, and slightly curved, were 2.3 mm long and 0.9 mm in maximum diameter. They were laid over the semiliquid provisions. The two cells collected during February at Anillaco contained an egg and a larva respectively. The larva, whitish, immobile and curved was located over a layer of remaining provisions at the base of the cell.
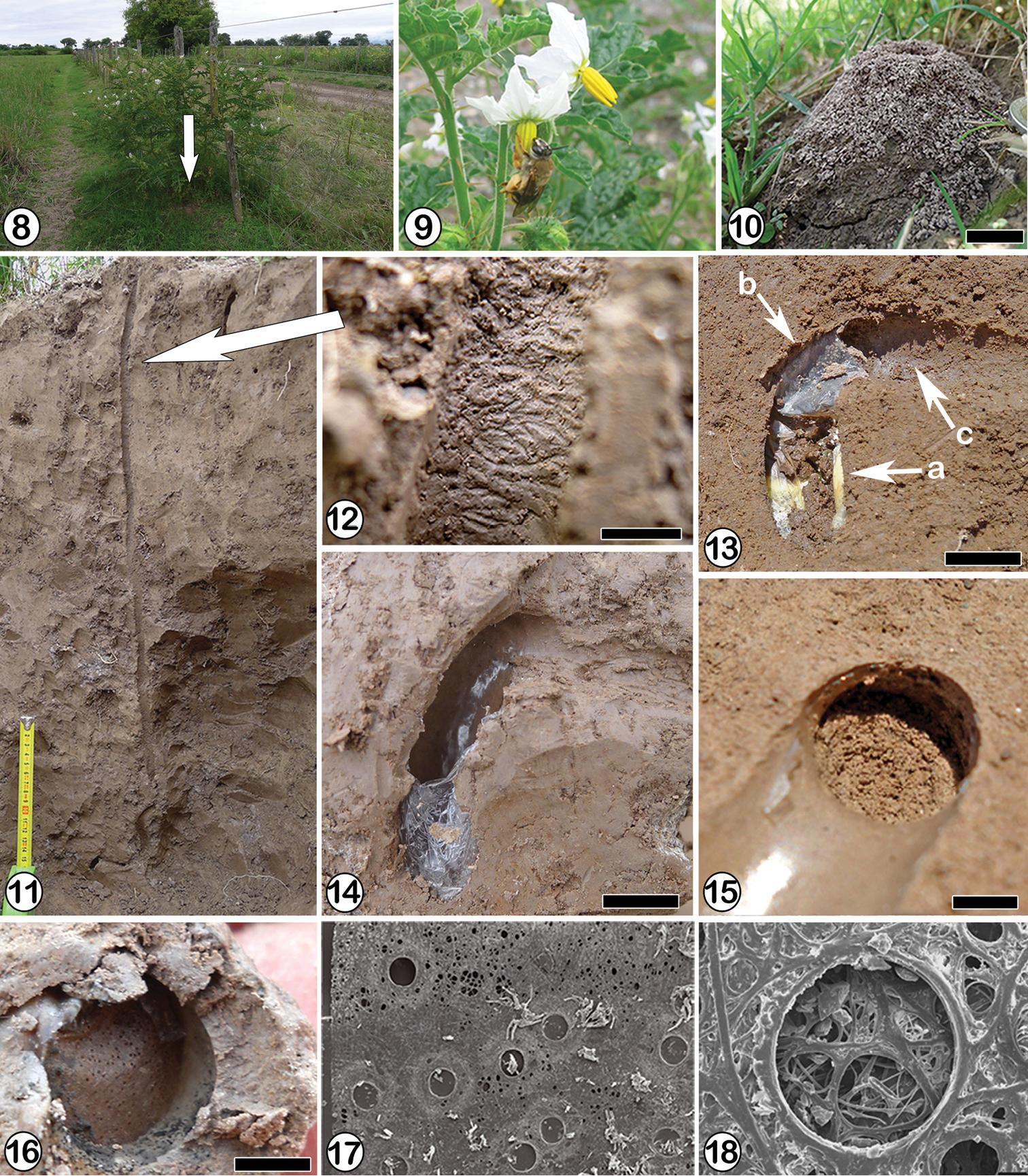
The observations on the biology and nest structure were carried out during January 25th–30th; March 10th–16th, 2011; and January 4th–14th, 2012 at Paraje La Florida (25°0.817'S, 65°33.534'W), Salta province, Argentina. Two nests were excavated at this locality, which is in a transitional habitat between rain and dry forest with a MAT around 17 C° and a MAP between 700 to 800 mm (
Ptiloglossa tarsata (Friese, 1900). 8 General view of the nesting site at “La Florida” (Salta province), the arrow indicates the location of the nest; 9 Female of Ptiloglossa tarsata foraging in a flower of Solanum sp. 10 Tumulus of unconsolidated soil, scale line: 1 cm 11 General view showing the nest architecture with a cell at the end of the main tunnel 12 Group of scratches probably produced by female´s mandibles. The arrow indicates their location in the main tunne; 13(a) cell with cellophane-like lining and provisions, (b) neck and (c) entrance tunnel, scale line: 1 cm 14 One cell showing the cellophane-like lining on the wall, scale line: 1 cm 15 Spiral closure of one cell, scale line: 0.5 cm 16 Cocoon operculum with holes, scale line: 0.5 cm 17 Scanning electron micrograph of the cocoon operculum showing the fabric of silk threads with small circular holes, scale: 500 µm 18 Onecircular hole surrounded by silk threads, scale: 50 µm.
The foraging activity started about 07:00 am, with full daylight, when females were observed visiting flowers of Solanum sp. (Fig. 9). One female made successive foraging trips, remaining inside the nest around 10 minutes after each trip. Foraging trips lasted around 20 minutes. This activity continued until 02:00 pm when the female closed the entrance from inside. The females were inside the main tunnels when the nests were excavated.
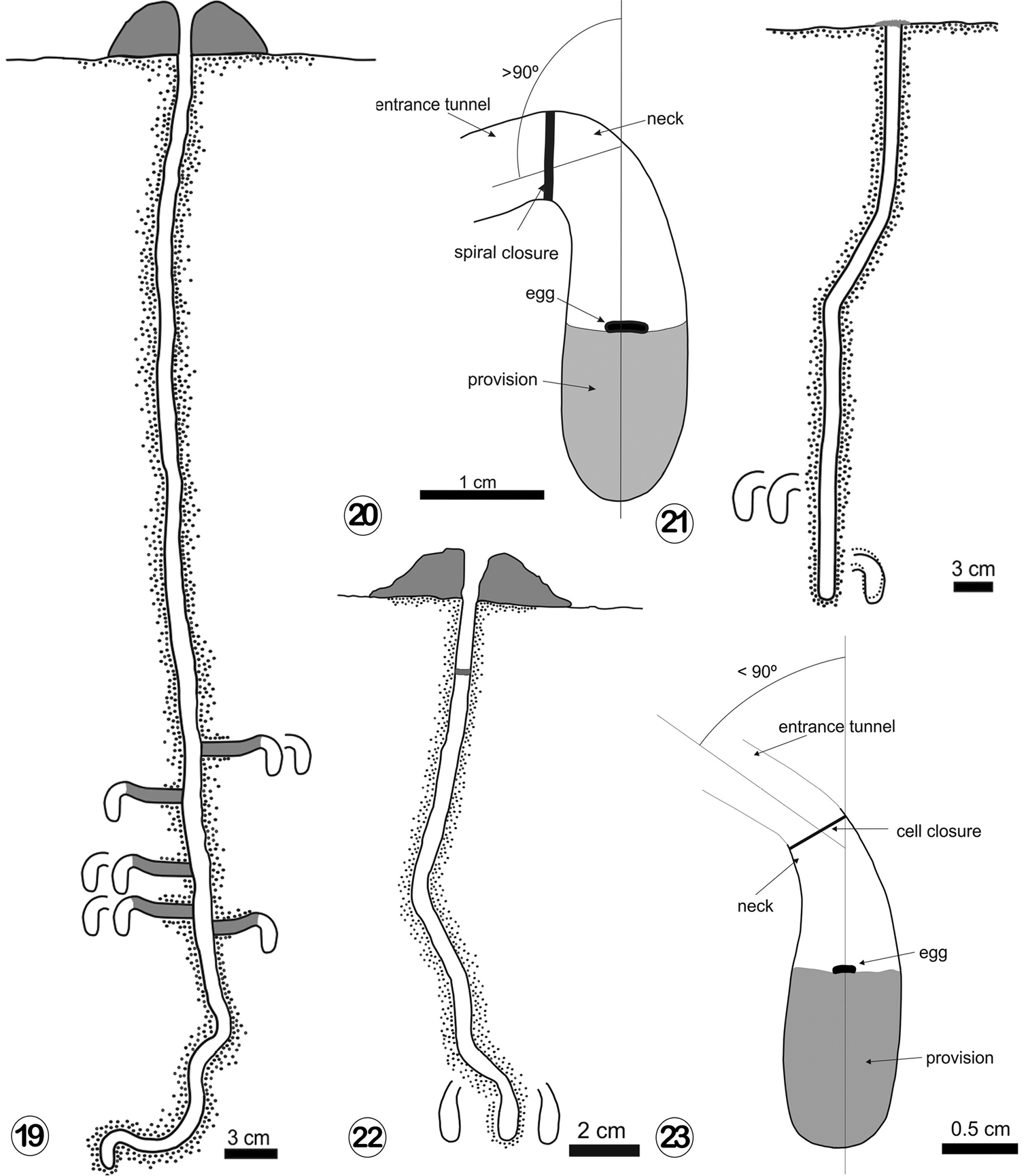
The entrance of the first nest, circular and 1 cm in diameter, was located at the center of a tumulus, 5.8 cm in maximum diameter and 4 cm high. The tumulus was composed of unconsolidated soil (Fig. 10). The main tunnel, vertical and slightly sinuous, was 40–61 cm long, and 1 cm in maximum diameter (Fig. 11). The inner surface of the main tunnel showed horizontal and densely grouped scratches 1–3 cm long and 0.2 cm wide. These scratches were probably made by the female mandibles (Fig. 12). The cells (n: 12), found at depths of 37–44 cm, were vertical and disposed radially around the main tunnel. Open cells were connected to the main tunnel by horizontal laterals, 5–7 cm long. Laterals ended in a raised, curved, entrance tunnel connecting with the vertical portion of the cell (Figs 11, 13c and 19). Once lined and sealed, the distal part of the entrance tunnel became the curved neck of the cell (Fig. 13b). Each lateral tunnel ended in one or two entrance tunnels connected with cells occurring at the same depth (Fig. 19). Entrance tunnels connected to closed cells were filled with soil (Fig. 19). The vertical portion of a cell was 1.9–2.0 cm long and 0.9–1 cm in maximum diameter (n: 12). The neck was 0.6–0.8 cm in diameter (n: 12). The inner walls of the cells and the neck were smooth and lined with a whitish, semitransparent cellophane-like material that extended up to the closure (Fig. 14). The cell closure, made with soil material, showed a spiral pattern composed of three coils on the inside (Fig. 15). Some cells contained a white wad of cotton-like material. The first nest excavated contained nine cells: three with eggs, three with young larvae, two with mature larvae, and one unfinished empty cell (Fig. 19). The second nest contained four cells, each with an egg. The eggs were whitish, cylindrical and lightly curved, 3 mm long and 0.8 mm wide. They lay on top of the semiliquid provisions (Fig. 20). Young larvae were also whitish, curved, and almost completely submerged in the provisions. Full grown larvae, more yellowish, were found inside cocoons made of a brown, thin, translucent, and slightly coriaceous material. The cocoon operculum is located at the base of the curved neck and consists of a circular disk of translucent, amber, semi-rigid material (Fig. 16). Below the operculum were two more disks of similar structure and composition to it. SEM observations indicated that these three disks were woven structures composed of crossed, coalescent silk threads of different thickness that leave small circular holes of 0.14–0.15 mm in diameter among them (Figs 17 and 18).
19 Nest architecture of Ptiloglossa tarsata (Friese, 1900). 20 Cell with provisions and egg, cell neck, spiral closure, and entrance tunnel of Ptiloglossa tarsata 21 Nest architecture of Ptiloglossa matutina (Schrottky, 1904) 22 Nest architecture of Cadeguala albopilosa (Spinola, 1851) 23 Cell with provisions and egg, cell neck, location of the cell closure and entrance tunnel of Cadeguala albopilosa.
Observations were carried out during 2012 on March 11th and November 10th in the Karadya Bioreserve (25°52.233'S, 53°58.167'W), near Andresito, Misiones province, Argentina (Fig. 24). The locality is included in the Upper Paraná Atlantic Forest Region. The climate is warm subtropical, without a dry season, MAT around 20° C and a MAP around 2000 mm (Servicio Meteorológico Nacional, 2012). During March, 11th, males of Ptiloglossa matutina were collected, probably before their emergence, inside tunnels exposed in a soil vertical section. On November 10th, one nest was excavated in a patch of lateritic soil, altered by human activities, containing abundant roots, litter, and some rocks.
Ptiloglossa matutina (Schrottky, 1904). 24 General view of the nesting site at Reserva Karadya, Andresito (Misiones province) 25 Nest entrance closed by a plug of soil(arrow), scale line:1 cm 26 Soil with roots, litter, some rocks, and remains of the main tunnel (arrow) 27 Cell showing the larva partially submerged in provisions, cellophane lining, and the wad cotton-like material attached the cell closure, scale line: 0.5 cm 28 Detail of the cell closure with the cotton-like material 29 Cell and neck wall with the lining removed. Note the high curvature.
The nest was excavated at midday and the female was found inside the main tunnel. Some weeks ago, similar nests were observed with the entrance open and females flying close to them after 06:00 pm (Julián Baigorria, pers. comm.).
The entrance was circular, 0.7 cm in diameter, without tumulus, and closed by a soil plug (Fig. 25). The main tunnel was circular in cross section, 25 cm long, nearly straight and slightly inclined downwards (Figs 21 and 26). It was 0.6–0.7 in maximum diameter. The nest contained four closed cells. One pair at a depth of 20 cm, were possibly connected to the main tunnel by a common tunnel filled with soil, 3 cm long. The other two cells, at a depth of 26 cm, were located at the other side of the main tunnel and also 3–4 cm of it (Fig. 26). The cells were vertical, rounded at the bottom and the neck was strongly curved (Figs 27 and 29). The vertical portion of the cell was 2.3 cm long and 1.3 cm in maximum diameter (n: 4). The neck was 0.8 cm in diameter (n: 4). The inner surface of cells and the neck were lined with a whitish semitransparent, cellophane-like material (Figs 27 and 28). Three cells contained eggs and one a young larva (Figs 27 and 28). The eggs, whitish, cylindrical and lightly curved, were 2.8 mm long and 0.4 mm wide. They laid on top of the yellow semiliquid provisions. The young larva was also whitish, curved, and almost submerged in the provisions. An earthen cell closure was not observed. Instead, a closure of white, cotton-like material that seems to be spirally arranged was observed (Fig. 28).
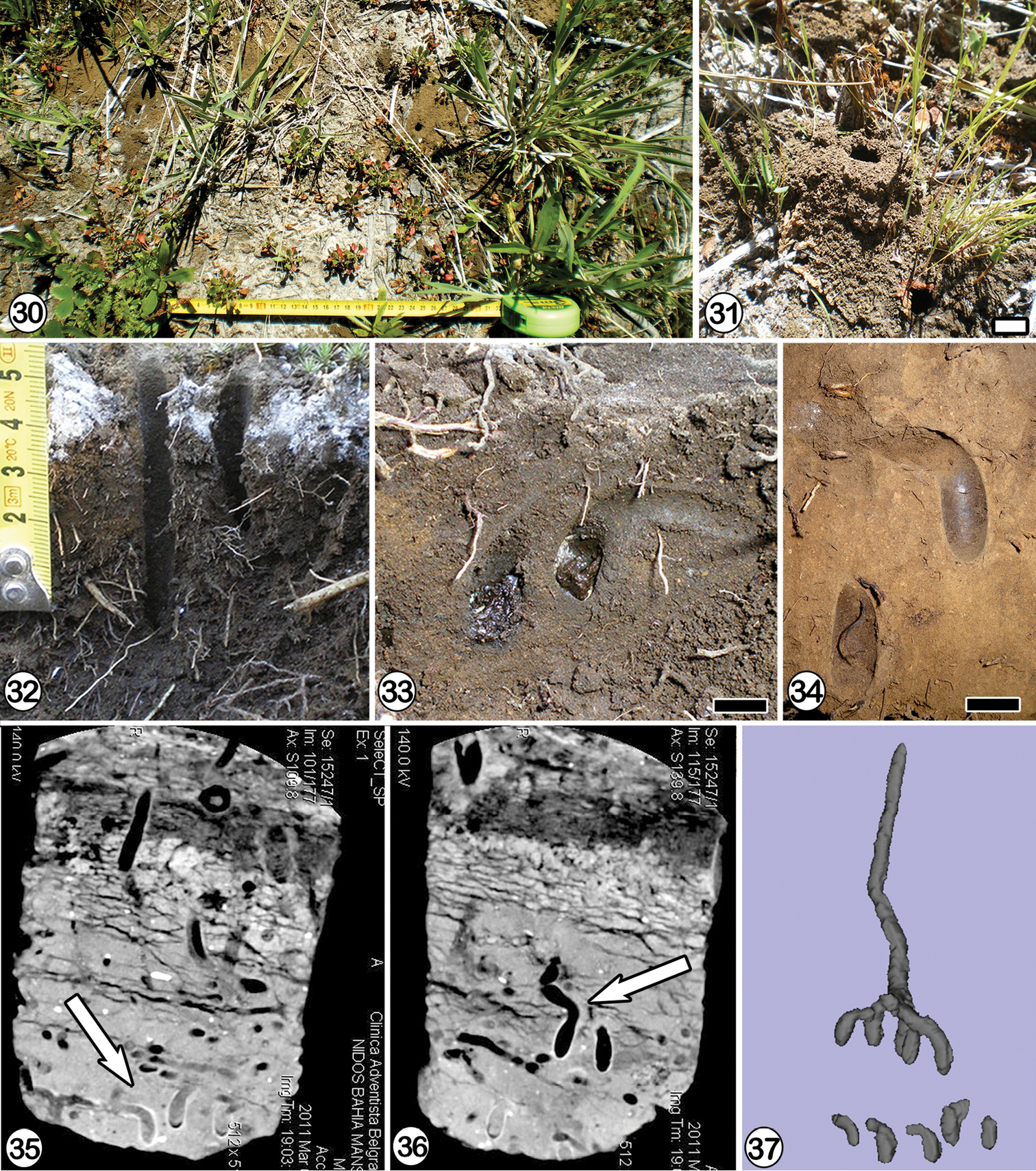
The observations were carried out during November, 4th, 2009 and February, 17th, 2011 at Bahia Mansa (43°7.467'S, 71°39.95'W), Parque Nacional Los Alerces, Chubut province, Argentina (Fig. 30). On November, 9th, 2009 a nesting site was also found along the route 235 (43°26.142'S, 72°10.017'W), near Yelcho Lake, Palena province (Region de Los Lagos), Chile. The nests were studied at the xeric Austrocedrus forest of Bahia Mansa (Parque Nacional Los Alerces) with a MAT around 8 C° and MAP around 1200 mm. The nesting site at Bahia Mansa was slightly sloped and the soil subsurface contained a thin ash layer produced by the Chaiten eruption of May 2008 (Figs 30 and 32). The soil cover was composed of grasses and short herbs (Figs 30 and 31). The nests, around 20 and located among grasses, were distributed in an area of 5 × 7 m. The soil, containing many rocks, roots and grass rhizomes, was composed of silt to very fine sandy material. Nests of sweat bees, aestivation chambers of earthworms, and feeding chambers of cicadas were also present. The Chilean locality along route 235 was a disturbed forest clearing composed of a dense grass cover. The area had some slopes with large rocks and trunks over the surface. Around 12 nests were distributed in an area of 6 × 1.5 m almost horizontal and partially covered by grasses. At both localities, closest nests were separated each other by 2–4 cm.
Cadeguala albopilosa (Spinola, 1851). 30 General view of the nest site at Bahia Mansa, Parque Nacional Los Alerces (Chubut province) 31 tumulusof unconsolidated soil32 two main tunnels and longitudinal view of the soil containing a thin ash layer 33 a pair of cells with provisions, necks and lateral tunnels, scale line: 1 cm 34 remains of cells of other nest, scale line: 1 cm 35–36 tomography images of one block of soil containing Cadeguala albopilosa nests, arrows indicate cells 37 3D-reconstruction of one nest and isolate cells.
Beeswere observed removing soil from the nests during November, 4th 2009 from 09:00 am to 05:00 pm. In the second field trip, during February 2011, no activity was observed.
Three nests were excavated during November 2009 when bees were active. The entrance, circular, 0.5 cm in maximum diameter was at the center of a large unconsolidated tumulus (Fig. 31). It was composed of a mixture of soil material and ash, 3–3.5 cm in maximum diameter and 2.5 cm high. During February 2011, when no activity of bees was observed, a piece of soil from the nesting site was extracted and taken to the laboratory for examination with a tomograph. The tomographic images provided a more precise picture of the nests and cells (Figs 35–37). The main tunnel, circular in cross section, was plugged with soil 2–3 cm below the entrance and descended vertically, straight or more sinuously among rocks (Fig. 22). It was 18–20 cm long and 0.5 cm in maximum diameter. The cells, found at a depth of 18–20 cm, were vertical and connected to the main tunnel by lateral ones, which were 2.5–3 cm long and mainly horizontal or gently curved (Fig. 23). They were filled with soil when connected to closed cells. At the distal end, lateral tunnels curved downwards, 90° or less, and were connected with the vertical portion of the cell. Once lined and sealed, the more distal curved part of the lateral tunnel connected to the cell became its curved neck (Figs 33 and 34). Each lateral tunnel ended in one or two cells ocurring at the same depth (Fig. 22). The number of cells per nest ranged from 2 to 4 in the studied nests (n: 4). The vertical portion of the cells was 1.3–1.6 cm long and 0.6–0.9 cm in maximum diameter (n: 12). The neck was 0.5 cm in diameter (n: 5). The inner walls of the cells and the neck were smooth and lined with a whitish semitransparent cellophane-like material. Cells studied on November contained eggs laying on the surface of the semiliquid provisions. The eggs whitish, cylindrical and slightly curved were 2 mm long and 0.7 mm wide. Cells studied on February contained white pupae inside their cocoons. The cocoons were composed of a brown, translucent, thin, and slightly coriaceous material. The cocoon operculum, located at the base of the curved neck, was composed of one translucent, amber, and semi-rigid circular disk, 5.0–5.5 mm in diameter (n: 2). This disk was a woven structure composed of crossed silk threads of different thickness that leave small circular holes. Below the operculum there was a net of silk threads loosely arranged, similar to the filter-like structure observed by
The observations were carried out during November 8th, 2009 and February 10th, 2011 beside the Río Negro Bridge (42°57.433'S, 72°39.233'W) and during November 9th, 2009 and February, 15th, 2011 at Lonconao (43°13.007'S, 71°55.143'W), both localities from the Palena province (Region de Los Lagos, Chile). The nesting sites occur in the glades of hygrophilous evergreen forests with a MAT around 11° C and MAP around 2500–3000 mm. Two nests were excavated. The first nest was located in a steep slope in a farm beside the Río Negro Bridge (Fig. 38), whereas the second was excavated in a low vertical section of the soil in another farm at Lonconao (Fig. 39). At both localities, the soil, composed of silty to very fine sandy material and devoid of rocks, contained grass rhizomes, some roots, and earthworm burrows. The soil cover consisted of a combination of dense grasses and dicots (Fig. 38), and the subsurface contained a thin ash layer produced by the Chaiten eruption of May 2008.
Diphaglossa gayi Spinola, 1851. 38 General view of the nesting site beside the Río Negro Bridge, Chile 39 general view of nesting site at Lonconao, Chile 40 tumulus of soil pellets and open entrance 41 main tunnel 42 nest architecture 43 cell, neck with lining, and egg laying on provisions.
On November 8th, 2009 the first nest was found around 06:00 pm and the female was observed entering the nest with pollen around 07:00 pm. On November 9th, 2009 the second nest was found also around 06:00 pm and the female was inside the main tunnel.
The Rio Negro nest showed an open circular entrance, 0.5 cm in diameter, which was surrounded by an eccentric tumulus, roughly 4.6 cm wide and 5.2 cm long. The tumulus was composed of soil pellets, probably of the recently deposited subsurface ash layer, which were paler than the soil surface (Fig. 40). The Lonconao nest was located in a vertical cut, thus the entrance, 0.7 in diameter, lacked a tumulus. Both nests share the same general structure. The main tunnel, circular in cross section, 32–38 cm long, was nearly straight and slightly inclined downwards, ending in a vertical segment 8 cm long (Figs 41 and 42). Its maximum diameter was 0.7–1.0 cm. Each nest contained four closed cells arranged in two pairs, one pair near the middle portion of the main tunnel, and the other near the end. It is possible that both cells were connected to the main tunnel by a common lateral, filled with soil when the nest was excavated (Fig. 42). The cellswere vertical, rounded at the bottom, and the neck was strongly curved (Figs 42 and 43). The vertical portion of the cells was 2 cm long and 1 cm in maximum diameter (n: 8). The neck was 0.7 cm in diameter. The inner surface of cells and the neck was lined with a whitish semitransparent, cellophane-like material. The cells in both nests contained one egg laying on top of the semiliquid provisions. The cell closure was not observed.
The five species studied herein share many ecological preferences, behaviors and features of nest architecture with each other, and with other Diphaglossinae, although some significant differences were also found during this study.
The broad ecological preferences of Diphaglossinae differ greatly among species as shown by its extended geographical distribution (
Diphaglossinae were considered traditionally as dim-light bees (
The proposed advantages of colleting pollen during twilight include: 1) reduction of competition for resources, 2) reduction of predators 3) reduction of nest parasites (e.g.
Some aspects of the nest architecture, as the curvature of entrance tunnels and cell necks, were proposed as advantages to face floodings. For example,
There is a tendency of Diphaglossinae species to nest in aggregations of few to many bees (
A tumulus, mostly concentric, surrounded the nest entrances in species studied herein that nested in horizontal surfaces as Zikanapis tucumana, Ptiloglossa tarsata, and Cadeguala albopilosa. Zikanapis tucumana was the only species that constructed a consolidate tumulus, also observed in Ptiloglossa arizonensis Timberlake by
The nest architecture of the species studied here was mostly similar to other Diphaglossinae described in the literature. The main and lateral tunnels were unlined and lack any particular surface texture in all species studied, with the exception of the main tunnel in nests of Ptiloglossa tarsata, which showed transverse scratches, probably produced by the female mandibles (Fig. 12).
Depending on the species studied herein, from the main tunnel arose 1 to 9 horizontal lateral tunnels connected with one cell as in Zikanapis tucumana or two cells as in Ptiloglossa tarsata, Ptiloglossa matutina, Cadeguala albopilosa, and probably Diphaglossa gayi. The presence of two cells connected to the same lateral is a novel feature for Diphaglossinae. In the five species studied here, the lateral tunnels were filled with soil when connected to closed cells.
The cell earthern closure of Diphaglossinae nests appears to be similar in all species, as in many bees, showing internally a spiral design (
The Diphaglossinae are the only group among the Colletidae whose larvae spin cocoons (
We thank Arturo Roig Alsina and Luis Compagnucci for the identification of the bees. Daniel Speranza, Martin Umazano, and Ricardo Melchor assisted us during the field trips to Vinchina and Chile. Mirta González identified soil composition, Luis Torino from Paraje La Florida (Salta, Argentina), and Mr. Eliseo from Lonconao (Chile) allowed us to work in their properties. Julián Baigorria assisted us with field observations at the Bioreserva Karadya and Ernesto Krauczuk helped us in Misiones province. Mr. Alaniz, David Gorla and Patricio Fidalgo provided us accommodation in La Rioja. Sebastian Sosa and Jeremias Taborda assisted us with tomography images. We thank all of them. We thank two anonymous reviewers and the Editor for improving the original manuscript.This is a contribution of PICT 1972 from the Agencia Nacional de Promoción Científica y Tecnológica of Argentina to Jorge F. Genise, and PIP 6023 and PIP 80100164 from CONICET to Ricardo Melchor.