| Taxon names | Citations | Turn highlighting On/Off |







(C) 2013 Andrew F. Ernst. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Ernst AF, István Mikó I, Deans AR (2013) Morphology and function of the ovipositor mechanism in Ceraphronoidea (Hymenoptera, Apocrita). Journal of Hymenoptera Research 33: 25–61. doi: 10.3897/JHR.33.5204
The ovipositor of apocritan Hymenoptera is an invaluable source of phylogenetically relevant characters, and our understanding of its functional morphology stands to enlighten us about parasitoid life history strategies. Although Ceraphronoidea is one of the most commonly collected Hymenoptera taxa with considerable economic importance, our knowledge about their natural history and phylogenetic relationships, both to other apocritan lineages and within the superfamily itself, is limited. As a first step towards revealing ceraphronoid natural diversity we describe the skeletomuscular system of the ceraphronoid ovipositor for the first time. Dissections and Confocal Laser Scanning Microscopy 3D media files were used to visualize the ovipositor complex and to develop character concepts. Morphological structures were described in natural language and then translated into a character-character state format, whose terminology was linked to phenotype-relevant ontologies. Four unique anatomical phenotypes were revealed: 1. The first valvifer (gonangulum) of the genus Trassedia is composed of two articulating sclerites, a condition present only in a few basal insect taxa. The bipartition of the first valvifer in Trassedia is most likely secondary and might allow more rapid oviposition. 2. Ceraphronoids, unlike other Hymenoptera, lack the retractor muscle of the terebra; instead the egg laying device is retracted by the seventh sternite. 3. Also unlike other Hymenoptera, the cordate apodeme and the anterior flange of the second valvifer are fused and compose one ridge that serves as the site of attachment for the dorsal and ventral T9-second valvifer muscles. Overall, the ceraphronoid ovipositor system is highly variable and can be described by discrete, distinguishable character states. However, these differences, despite their discrete nature, do not reflect the present classification of the superfamily and might represent parallelisms driven by host biology.
Skeletomusculature, second valvula, ovipositor sheath, Aphanogmus, Ceraphron, Conostigmus, Megaspilus, Dendrocerus, Lagynodes, Hymenoptera Anatomy Ontology, Phenotypic Quality Ontology, Spatial Ontology
Ceraphronoidea has, until recently, been one of the most neglected groups of parasitic Hymenoptera, despite their ubiquity in the environment and fascinating life history strategies, which span from primary to quaternary parasitism (hyper-hyper-hyperparasitism) (
The phylogenetic placement of Ceraphronoidea remains uncertain despite the recent efforts to resolve the Hymenoptera tree of life (
The most comprehensive review on the classification of the superfamily has proposed by Dessart and Cancemi (1987). Although this classification served as a template for further studies (Masner 1993,
It is evident that a comprehensive study using both morphological and molecular characters is necessary for the reevaluation of Ceraphronoidea systematics. Since traditionally used morphological characters have been phylogenetically inconsistent the utilization of unexplored character systems, such as the male and female terminalia might offer additional data relevant to more robustly estimate the phylogenetic history of this group.
Despite the extensive descriptive work done on comparative morphology of the Hymenoptera female terminalia (
Specimens used in this study (Table 1) were identified by Andrew Ernst (AFE) and István Mikó (IM). Megaspilinae was represented in our study by Megaspilus armatus (Say), Dendrocerus spissicornis (Hellén) and Conostigmus abdominalis (Boheman); Lagynodinae (Megaspilidae) by Lagynodes sp. (AFE); and Ceraphronidae by Ceraphron sp. (IM) (Fig. 5), Aphanogmus sp.1 (AFE), Aphanogmus sp. 2 (IM) and Trassedia luapi (Cancemi).
Specimens observed for morphological description including specimen identifiers, preparation techniques, imaging method and collecting locality (verbatim collecting data are stored at fighsare.com).
| Taxon | Specimen identifier | Imaging techniques | Locality | DOI of CLSM media files stored at http://figshare.com |
|---|---|---|---|---|
| Aphanogmus sp. 1 | NCSU 0055648 | brightfield | Hungary | |
| Aphanogmus sp. 1 | NCSU 0002419 | CLSM 40× water imersion | Hungary | https://doi.org/10.6084/m9.figshare.156446 https://doi.org/10.6084/m9.figshare.156439 |
| Aphanogmus sp. 2 | PSUCIM_5009 | brightfield | Madagascar | |
| Ceraphron sp. | NCSU 0071198 | brightfield | Madagascar | |
| Ceraphron sp. | NCSU 0071197 | brightfield, CLSM 10x | Madagascar | https://doi.org/10.6084/m9.figshare.156445 |
| Ceraphron sp. | PSUCIM_5005, 5006 | CLSM | Madagascar | https://doi.org/10.6084/m9.figshare.156470 https://doi.org/10.6084/m9.figshare.156469 https://doi.org/10.6084/m9.figshare.156447 |
| Conostigmus abdominalis (Boheman) | NCSU 0056302 | brightfield | Sweden | |
| Conostigmus abdominalis (Boheman) | NCSU 0056301 | brightfield | Sweden | |
| Conostigmus abdominalis (Boheman) | NCSU 0055647 | brightfield, CLSM 20× | Sweden | https://doi.org/10.6084/m9.figshare.156448 https://doi.org/10.6084/m9.figshare.156433 |
| Dendrocerus spissicornis Hellén | PSUCIM_5001, 5002 | CLSM | Sweden | https://doi.org/10.6084/m9.figshare.156452 https://doi.org/10.6084/m9.figshare.156451 https://doi.org/10.6084/m9.figshare.156450 https://doi.org/10.6084/m9.figshare.156449 |
| Dendrocerus spissicornis Hellén | PSUCIM_5010 | CLSM | Sweden | https://doi.org/10.6084/m9.figshare.156459 https://doi.org/10.6084/m9.figshare.156453 |
| Lagynodes sp. | NCSU 0055643 | brightfield | USA | |
| Lagynodes sp. | NCSU 0056306 | CLSM 20×, 40× water immersion | USA | https://doi.org/10.6084/m9.figshare.156454 https://doi.org/10.6084/m9.figshare.156443 |
| Lagynodes crassicornis | ||||
| Megaspilus armatus (Say) | NCSU 0071199 | brightfield | USA | |
| Megaspilus armatus (Say) | NCSU 0055645 | brightfield, CLSM 20× | USA | https://doi.org/10.6084/m9.figshare.156442 https://doi.org/10.6084/m9.figshare.156434 |
| Megaspilus armatus (Say) | NCSU 0056307 | CLSM 40× water imersion | USA | https://doi.org/10.6084/m9.figshare.156437 |
| Megaspilus armatus | PSUCIM_5003 | CLSM 20X | Canada | https://doi.org/10.6084/m9.figshare.156458 https://doi.org/10.6084/m9.figshare.156457 https://doi.org/10.6084/m9.figshare.156456 https://doi.org/10.6084/m9.figshare.156455 |
| Trassedia luapi Cancemi | NCSU 0056318 | brightfield, CLSM 10× | Madagascar | https://doi.org/10.6084/m9.figshare.156440 https://doi.org/10.6084/m9.figshare.156438 https://doi.org/10.6084/m9.figshare.156436 |
| Trassedia luapi Cancemi | NCSU_0071196 | brightfield, CLSM 20× | Madagascar | https://doi.org/10.6084/m9.figshare.156467 https://doi.org/10.6084/m9.figshare.156444 |
Specimens used in the present study were stored in 95% ethanol. Some specimens were critical point dried and dissected on Blue-Tack (Blue Tack, Bostik inc.) medium. This method is mostly used to reveal the spatial relationships between muscles. Other specimens were dissected in glycerin on a concave microscope slide or were macerated in KOH to visualize the skeletal structures. Dissections and observations were made using an Olympus SZX16 stereomicroscope and an Olympus CX41 compound microscope.
Bright field images were taken using an Olympus CX41 compound microscope, equipped with an Olympus DP71 digital camera. Image stacks were combined using CombineZP (
To verify the relationships between anatomical structures, serial transverse sectioning was carried out on a Lagynodes specimen (Table 1). The specimen was embedded in Araldit®, cut at 1 µm with a Microm microtome (HM 360), and stained with toluidine blue.
Anatomical terms used in the descriptions are linked to concepts in the Hymenoptera Anatomy Ontology (HAO; Hymenoptera Anatomy Portal (http://portal.hymao.org),
Verbatim descriptions composed in natural language serve as the traditional way for communicating observations in insect morphology. However, these descriptions can be decoded only by morphology experts, are hardly accessible to non-expert researchers and cannot be reasoned over efficiently by text-mining applications (
To meet the grand challenge of describing phenotypes in a semantic way, using Web Ontology Language (OWL; http://www.w3.org/TR/owl-features/) for example, one must be familiar with tools of the Semantic Web (e.g., Protégé, http://protege.stanford.edu/ and Manchester Syntax, http://www.w3.org/TR/owl2-manchester-syntax/). Perhaps more importantly, one also has to provide a character/character state description with terms explicitly linked to ontologies.
We provide here an example of the transformation of our natural language descriptions to character/character state format and to link the terminology to relevant phenotype ontologies. Our goal is to make this product more accessible to future reasoning applications. During the “ontologization” procedure the describer is forced to provide strict, structure-based definitions for each anatomical concept, which itself enhances the readability, objectivity, consistency and comparability of the research product.
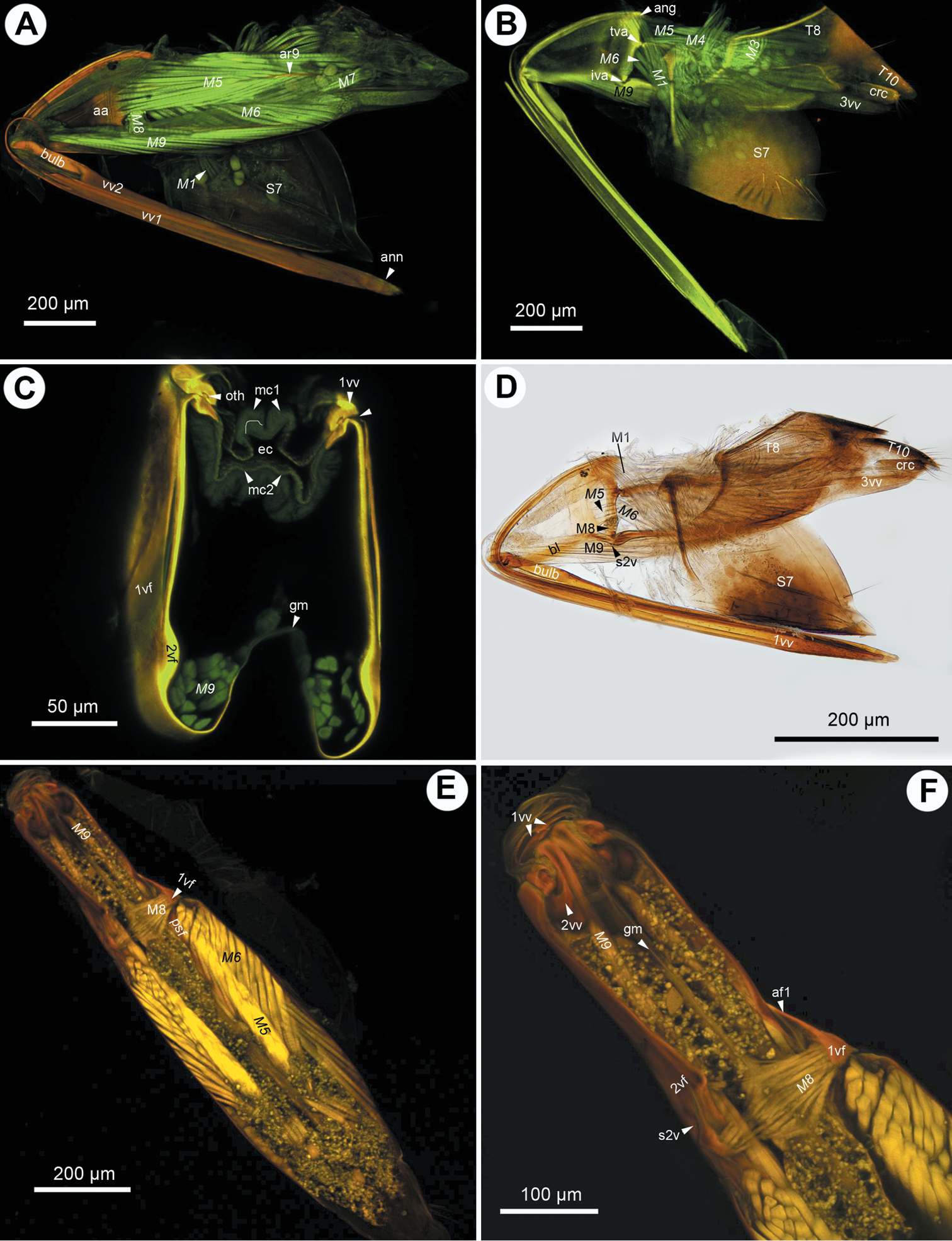
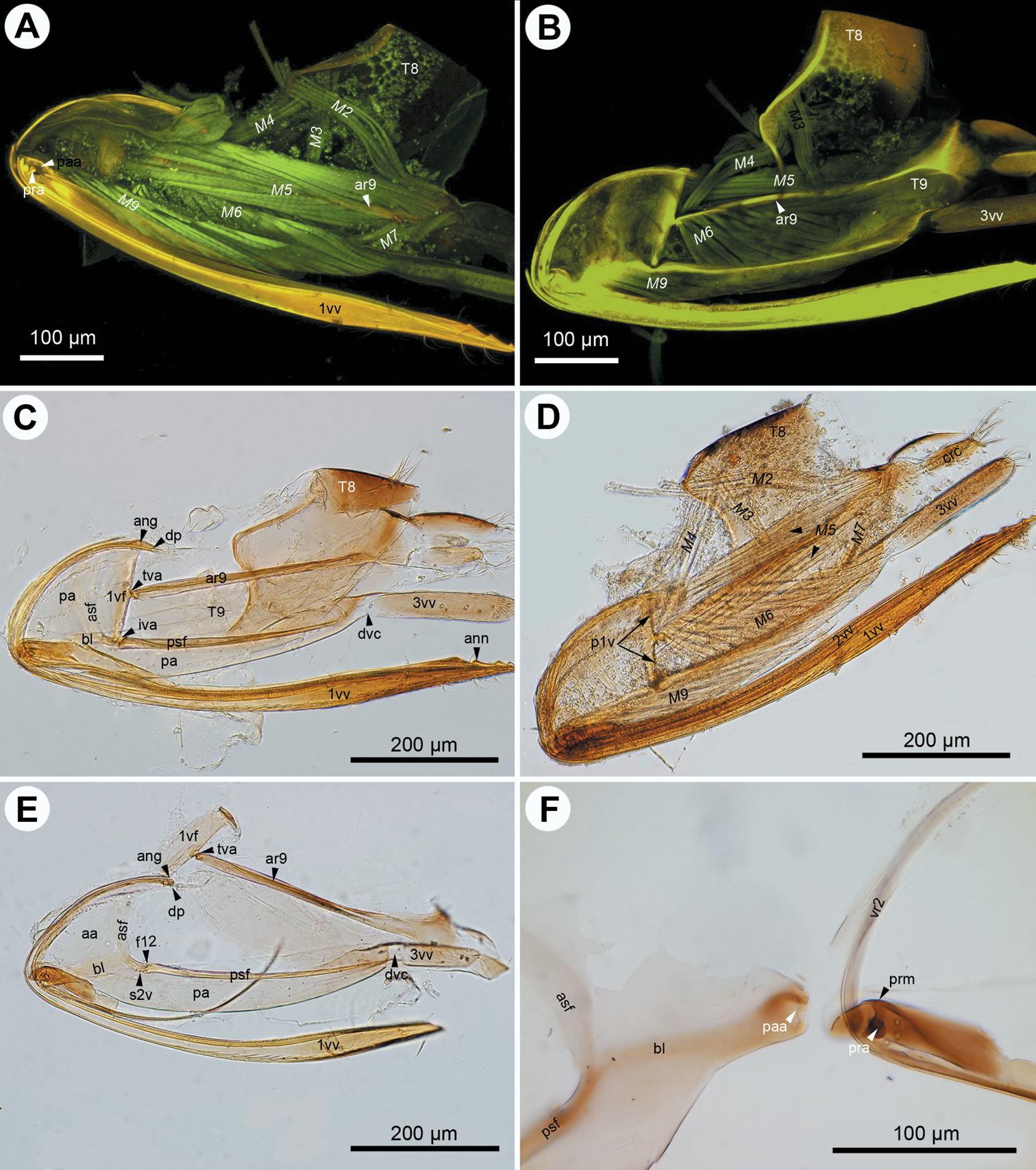
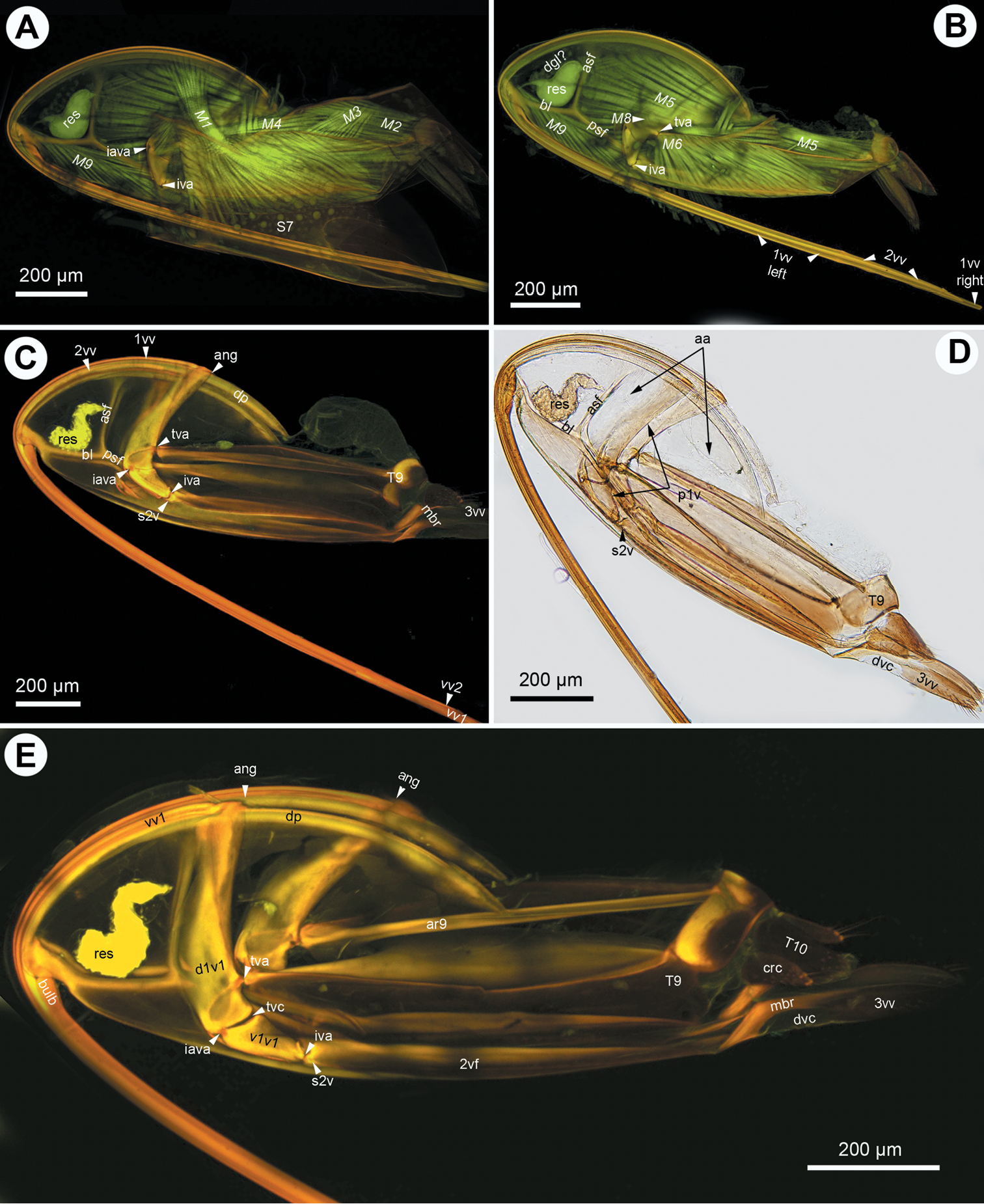
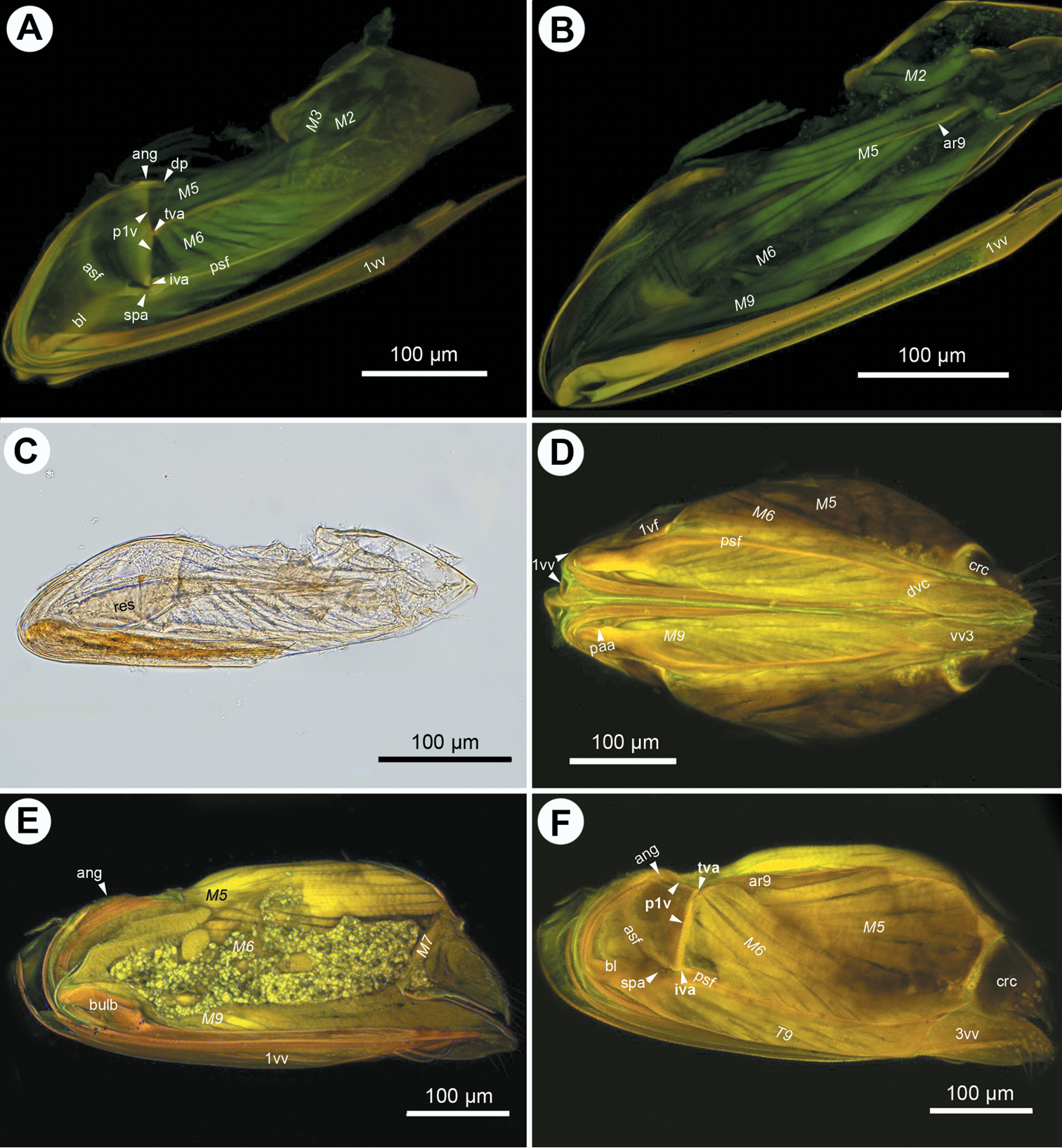
The first valvifer is dorsoventrally elongated in lateral view (ch1: 0; 1vf: Figs 2E, 5A, E, 6A, D) with convex anterior (ch2:0) and straight (ch3:0; p1v: Figs 2D, 4A, 6A) or concave posterior margins (ch3:1; p1v: Fig. 3D) in all taxa except Dendrocerus where the posterior margin of the first valvifer is angled at the tergo-valviferal articulation (ch3:2; p1v: Fig. 4F). The first valvifer is not subdivided (ch4:1; 1vf: Figs 2E, 5A, E, 6A, D) except in Trassedia, where the transvalvifer conjunctiva (ch4:0; tvc: Fig. 3E) separates the elongate dorsal sclerite (ch5:0; d1vf: Fig. 3E) from the triangular ventral sclerite of the first valvifer (ch6:0; v1vf: Fig. 3E). The sclerites articulate with one another at the intravalvifer articulation (ch7:0; iava: Figs 3C, E), which is located anteriorly on the border between the two sclerites (ch8:0). The ventral margin of the dorsal sclerite (ch9:0) and the anterodorsal margin of the ventral sclerite are thickened relative to surrounding regions (ch10:0). The anterior flange of the first valvifer (af1: Fig. 1F) overlapping the second valvifer is present (ch11:0) in Conostigmus, Dendrocerus and Megaspilus, and Lagynodes but absent from Ceraphronidae (ch11:1). The first valvifer articulates with the second valvifer on its posteroventral corner (ch12:0; intervalvifer articulation, iva: Figs 1B, 2C, 3A–C, E, 4A, F, 5A, B, E, 6A, C, D) and with T9 on its posterior margin (ch13:0; tergo-valvifer articulation, tva: Figs 1B, 2C, 3B, C, E, 4A, F, 5A, B, E, 6A, C, D). The tergo-valvifer articulation is in the middle of the posterior margin of the first valvifer in Conostigmus and Lagynodes (ch14:0; tva: Figs 2C, 4A), it is on the ventral half of the margin (ch14:1; tva: Figs 3B, C, E, 5A, B, E, 6D) in Ceraphron, Aphanogmus sp. 2 and Trassedia, where it is located ventrally on the posterior margin of the dorsal sclerite of the first valvifer (ch15:0; tva: Fig. 3B, C, E), it is in the upper half of the margin in Megaspilus and Dendrocerus (ch14:2; tva: Figs 1B, 4F) and adjacent to the anterior angle in Aphanogmus sp. 1 (ch14:3; tva: Figs 6A, C).
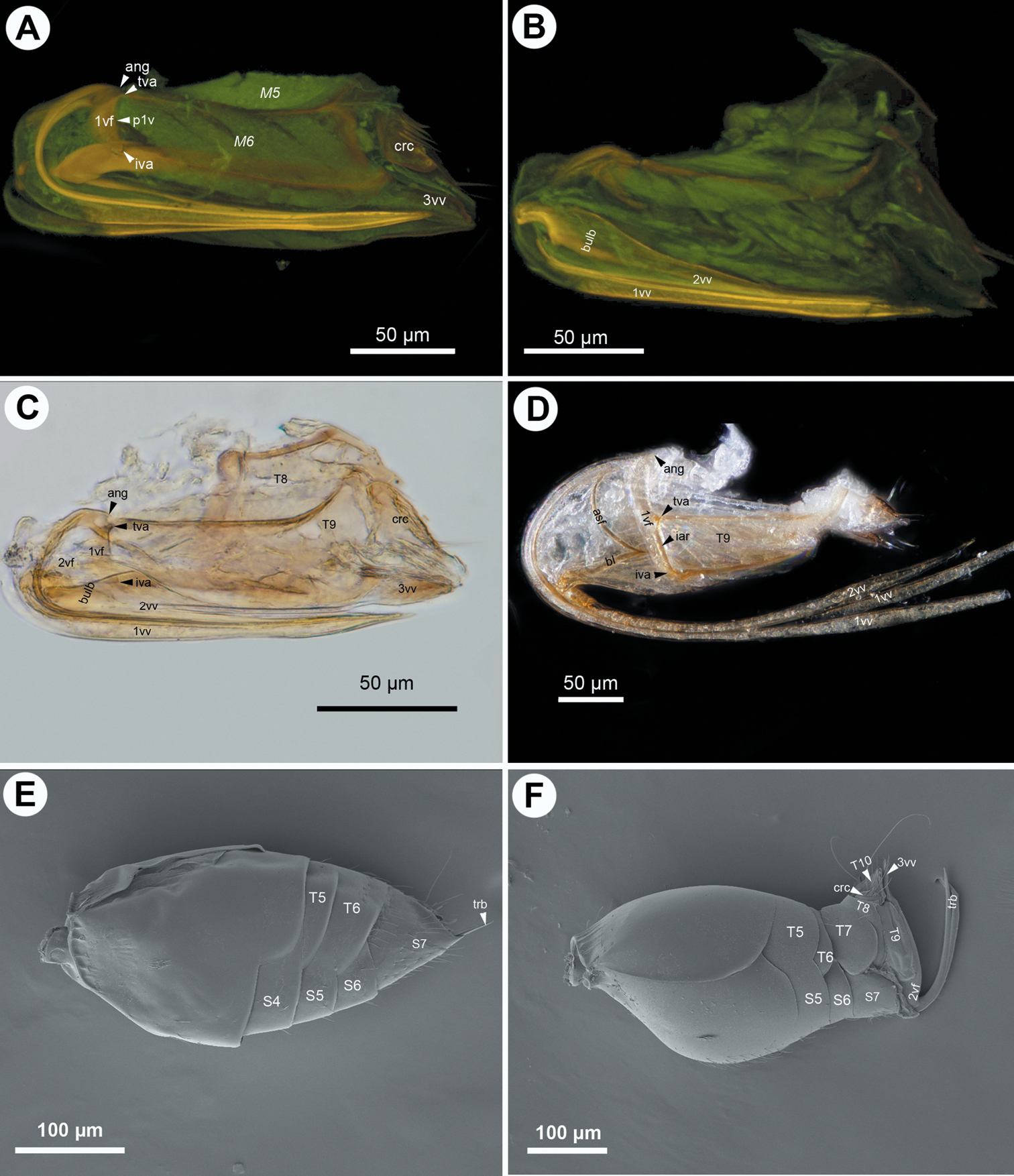
CLSM micrographs and bright field image showing the female terminalia of Megaspilus armatus (Say). A CLSM, medial view (doi: 10.6084/m9.figshare.156434) B CLSM, lateral view (doi: 10.6084/m9.figshare.156442) C CLSM, transverse section, white line indicates site of separation of median conjunctivae of the first valvulae (doi: 10.6084/m9.figshare.156437) D bright field, lateral view E–F CLSM, frontal section, dorsal view (doi: 10.6084/m9.figshare.156458; doi: 10.6084/m9.figshare.156457, doi: 10.6084/m9.figshare.156456, doi: 10.6084/m9.figshare.156455). All anterior to the left.
CLSM micrographs and bright field image showing the female terminalia of Conostigmus abdominalis (Boheman). A CLSM, medial view (doi: 10.6084/m9.figshare.156448) B CLSM, lateral view (doi: 10.6084/m9.figshare.156433) C bright field, medial view, muscles removed D bright field, medial view E bright field, medial view, first valvifer and T9 separated from second valvifer, muscles removed; F, bright field, second valvifer removed from second valvulae, muscles removed. All anterior to the left.
CLSM micrographs and bright field image showing the female terminalia of Trassedia luapi (Cancemi). A CLSM, lateral view, T8 intact (doi: 10.6084/m9.figshare.156440) B CLSM, lateral view, T8 removed (doi: 10.6084/m9.figshare.156438) C CLSM, lateral view, muscles removed (doi: 10.6084/m9.figshare.156444) D bright field, lateral view, muscles removed E CLSM, lateral view, muscles removed (doi: 10.6084/m9.figshare.156467). All anterior to the left.
CLSM micrographs and bright field image showing the female terminalia of Megaspilidae. A–C: Lagynodes sp.: A CLSM, lateral view (doi: 10.6084/m9.figshare.156443) B CLSM, medial view (doi: 10.6084/m9.figshare.156454) C bright field, medial view. D–F Dendrocerus spissicornis (Hellén), CLSM: D ventral view (doi: 10.6084/m9.figshare.156451, doi: 10.6084/m9.figshare.156449) E medial view (doi: 10.6084/m9.figshare.156452) F lateral view (doi: 10.6084/m9.figshare.156459, doi: 10.6084/m9.figshare.156450). All anterior to left.
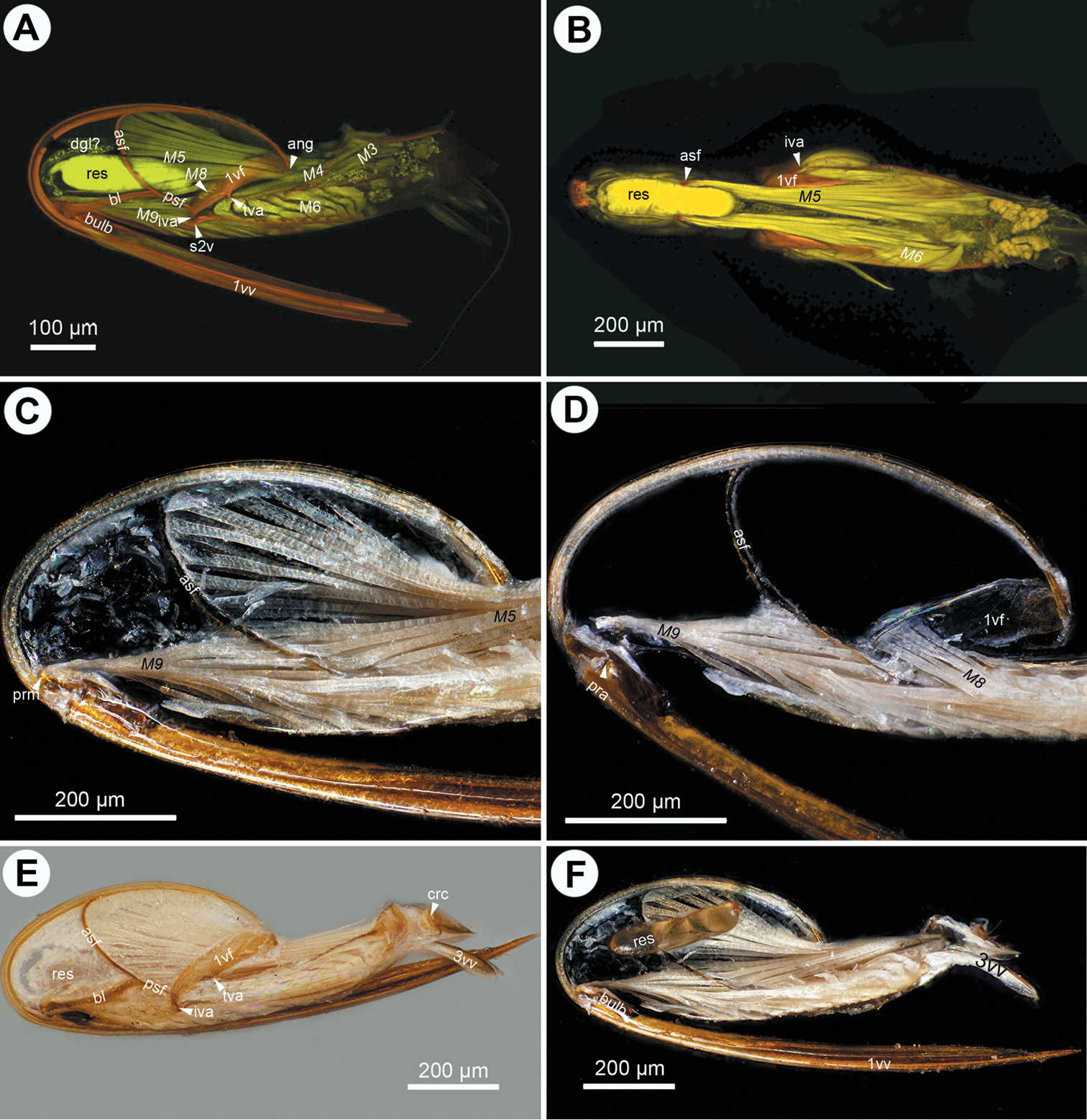
CLSM micrographs and bright field image showing the female terminalia of Ceraphron sp. A CLSM, lateral view (doi: 10.6084/m9.figshare.156445) B CLSM, frontal section, dorsal view (doi: 10.6084/m9.figshare.156447) C bright field, lateral view D bright field, medial view, dorsal T9-second valvifer muscle removed E bright field, medial view, dorsal T9-second valvifer muscle intact; F, bright field, medial view, venom gland reservoir intact. All anterior to left.
CLSM micrographs and bright field image showing the female terminalia of Ceraphronidae. A–C: Aphanogmus sp. 1: A CLSM, lateral view (doi: 10.6084/m9.figshare.156439) B CLSM, medial view (doi: 10.6084/m9.figshare.156446) C bright field, lateral view D Aphanogmus sp. 2, bright field, lateral view E Synarsis sp., SEM, lateral view F Cyoceraphron sp., SEM, lateral view. All anterior to left.
The anterior area of the second valvifer is expanded dorsally into a broad, flat, laterally directed surface (ch16:0; aa: Figs 1A, 2E). The basal line of the second valvifer (ch17:0) is a sharply defined ridge in Ceraphron, Trassedia, and Aphanogmus sp. 2 (ch18:0; bl: Figs 3B–D, 5A, E, 6D) but a thickening with diffuse margins in Aphanogmus sp. 1 and Megaspilidae (ch18:1; bl: Figs 1D, 2C, E, 4A, F). The dorsal projection of the second valvifer (ch19:0) is longer than the length of the anterior area of the second valvifer in Ceraphron and Trassedia, and Aphanogmus sp. 2 (ch20:1; dp: Figs 3C, E) whereas the projection is shorter than the length of the anterior area in other taxa examined (ch20:2. dp: Figs 2E, 4A). The anterior section of the dorsal flange of the second valvifer is sharply defined ridge in Ceraphron, Aphanogmus sp. 2, and Trassedia (ch21:0; asf: Figs 3B, C, D, 5A–E, 6D) but a thickening with diffuse margins in Megaspilidae and Aphanogmus sp. 1 (ch21:1; asf: Figs 2C, E, F, 4A, F). The posterior section of the dorsal flange of the second valvifer is sharply defined (ch22:0; psf: Figs 2C, E, 4A, D, 5A, E). The area of the second valvifer posterior to the intervalviofer articulation is elongate (ch23:0 pa: Figs 2C, E). The ventral margin of the second valvifer curves mediodorsally (ch24:0) encircling the posterior second valvifer-second valvula muscle (ch25:0. 2vf, M9: Fig. 1C). The genital membrane of the second valvifers accommodates the terebra when it is retracted (ch26:0; gm: Fig. 1C). The venom gland reservoir is surrounded by the second valvifer is present in Ceraphronidae and Lagynodes (ch27:0; res: Figs 3A–E). The reservoir was not observed in other Megaspilidae (ch27:1). The content of the reservoir is yellowish, transparent and hard, resin-like (ch28:0; res: Fig. 5F) in critical point dried specimens.
The first valvula tapers distally in lateral view in Ceraphron, Aphanogmus sp. 2 and Trassedia (ch29:0; 1vv: Figs 3B, 5A, F, 6D), whereas it is spatulate in Megaspilidae and Aphanogmus sp. 1 (ch29:1; 1vv: Figs 1A, D, 2A, C, D, E, 4A, B, 6B, C). The banding pattern and annuli are missing from the first valvula (ch30:0; ch31:0).
The second valvulae are expanded proximally into the bulb (ch37:0; bulb: Figs 1A, D) and fused distal to the bulb (ch35:0). The dorsal valve tapers distally in dorsal view (ch36:0) and the anterior margin of the bulb is curved dorsally. The processus articularis is located laterally (ch32:0; pra: Figs 2F, 5D), the anterior notch of the dorsal valve anteriorly (ch33:0) and the processus muscularis anterodorsally on the bulb (ch34:0; prm: Fig. 2F). The anterior area of the second valvifer is 2.0 times as high as the bulb in lateral view in Dendrocerus and Aphanogmus sp. 1 (ch38:0; Figs 4E, 6B) whereas it is more than 2.0 times as high as the bulb in Ceraphron and Megaspilidae (ch39:1). Banding pattern is absent (ch39:0) whereas annuli are present on the dorsal valve in Megaspilidae (ch40:0; ann: Figs 1A, 2C) but absent in Ceraphronidae (ch40:1). The number of annuli is four (ch41:0) in Conostigmus and Lagynodes and six (ch41:1) in Megaspilus.
The third valvula and the second valvifer are fused at the median bridge (ch42:0; ch44:0; mbr: Figs 3C, E) connecting the opposite second valvifers at their posterior ends. A ventral, vertical conjunctiva marks the site of fusion of the two sclerites (ch43:1; dvc: Figs 2C, E, 3D, E, 4D). The third valvula tapers distally in lateral view (ch45:0; 3vv: Figs 1B, 2B–E, 3C–E, 4D, F, 5E, F, 6A, B, F), its lateral wall is convex (ch46:0) and sclerotized (ch47:0) whereas its medial wall is concave (ch48:0; 3vv: Fig. 3E) and membranous (ch49: 0; 3vv: Fig. 3F).
T9 is quadrangular in lateral view (ch50:0; T9: Figs 2B, C) and wider than long dorsomedially (ch51:0; T9: Figs 3D, E). The cordate apodeme and the anterior flange are continuous, composing the anterior ridge of T9 (ch52:0; ar9: Figs 2A–C, 3E, 4B), which is adjacent anteriorly with and separated posteriorly from the anterior margin of T9 (ch53:0); the distance between the anterior margin of T9 and the ridge increases gradually posteriorly (ch54:0).
S7-first valvula muscle (M1: Figs 1B, D, 3A) arises anteriorly from S7 (ch55:0) and inserts on the first valvula at the dorsal margin of the ovipositor adjacent to the border of the first valvula and the dorsal end of the first valvifer (ch56:0). The muscle is oriented posterodorsally (ch57:0). The dorsal T8-T9 muscle (M2: Figs 2A, 3A, 4A, B) arises from along the anterior margin of T8 (ch58:0) and inserts dorsally on the anterior margin of T9 (ch59:0). The lateral T8-T9 muscle (M3: Figs 1B, 2A, B, D, 3A, 4A, 5A) arises from T8 posterodorsally of the dorsal T8-T9 muscle (ch60:0), and inserts on the anterior ridge of T9 (ch61:0). The T8-first valvifer muscle (M4: Figs 1B, 2A, 2B, 3A, 5A) arises from T8 dorsally of the site of attachment of the lateral T8-T9 muscle (ch62:0) and inserts on the first valvifer adjacent to the ninth tergal condyle of the first valvifer (ch63:0). The muscle inserts on the ventral sclerite of the first valvifer adjacent to the intravalvifer articulation (ch64:0; M4: Fig. 3A) in Trassedia. The dorsal T9-second valvifer muscle (M5: Figs 1A, B, D, E, 2A, B, D, 3B, 4A, B, D, E, F, 5A–C, 6A) arises dorsally and ventrally from the anterior ridge of T9 with sites of origins extending ventrally and dorsally on the wall of the tergite (ch65:0) and inserts along the posterior margin of the anterior area of the second valvifer (ch66:0). The ventral T9-second valvifer muscle (M6: Figs 1A, B, D, E, 2A, B, D, 3B, 4A, B, D, F, 5A, B, 6A) is not subdivided (ch67:0) and arises from the medial side of the posterior area of the second valvifer (ch68:0). The site of insertion of the muscle extends along the anterior part of the anterior ridge of T9 in most taxa examined (ch69:0) except Megaspilus, and Dendrocerus where the muscle seemingly inserts partly on the interarticular ridge of the first valvifer (ch70:0; M6: Figs 1B, D, 4F). The posterior T9-second valvifer muscle (M7: Figs 1A, 2A, D, 4E) arises from the strap-like dorsal area of T9 (ch71:0) and inserts on the median bridge (ch72:0). The fan shaped first valvifer-genital membrane muscle (ch73:0; M8: Figs 1A, D, E, F, 3B, 5A, D) arises from the medial surface of the first valvifer near the intervalvifer articulation (ch74:0.). The site of insertion of the muscle extends along the genital membrane (ch75:0). In Trassedia the muscle arises from the medial surface of the dorsal sclerite of the first valvifer (ch76:1; M8: Fig. 3B). The posterior second valvifer-second valvula muscle (M9: Figs 1A–F, 2A, B, D, 3A, B, 4B, D–F, 5A, C, D) arises from the medial side of the posterior area of the second valvifer (ch77:0), and inserts on the processus musculares (ch78:0). In Ceraphron and Trassedia some bands of the muscle arise from the anterior area of the second valvifer (ch.77:1; M9: Figs 3A, B, 5A, C, D). T9-genital membrane muscle (ch79:0), lateral T9-second valvifer muscle (ch80:0), second valvifer-genital membrane muscle (ch81:0) and the anterior second valvifer-second valvula muscles (ch82:0) are absent from Ceraphronoidea.
Anterior margin of first valvifer: shape
Posterior margin of first valvifer: shape
(2) bent at tergo-valvifer articulation
Transvalvifer conjunctiva: count
(0) present
(1) absent
Dorsal sclerite of the first valvifer: shape
Ventral sclerite of the first valvifer: shape
(0) triangular in lateral view
Intravalvifer articulation: count
(0) present
(1) absent
Intravalvifer articulation: position
(0) anterior region of first valvifer
Ventral margin of
(0) thickened
Anterodorsal margin of ventral sclerite of the first valvifer: thickness
(0) thickened
Anterior flange of the first valvifer: count
(0) present
(1) absent
Second valviferal condyle of the first valvifer: position
(0) at the posteroventral corner of the first valvifer
Ninth tergal condyle of the first valvifer: position
(0) posterior margin of the first valvifer
Distance between tergo-valvifer articulation and intravalvifer articulation (D1): proportion to the distance between tergo-valvifer articulation and anterior angle of first valvifer (D2).
(0) D1=D2
(1) D1<D2
(2) D1>D2
(3) D2=0
Ninth tergal condyle of the first valvifer: position
(0) on dorsal sclerite of the first valvifer
Anterior area of the second valvifer: height vs. height of posterior area of second valvifer
(0) 3≤:1
Basal line of the second valvifer: count
(0) present
Basal line of the second valvifer: sharpness
(0) sharp
(1) blunt
Dorsal projection of the second valvifer: count
(0) present
Length of dorsal projection of second valvifer in lateral view (L1) vs.
(0) L1<L2
(1) L1>L2
Anterior section of dorsal flange of the second valvifer//purl.obolibrary.org/obo/HAO_0002173>: sharpness
(0) sharp
(1) blunt
Posterior section of dorsal flange of second valvifer: sharpness
(0) sharp
Posterior area of second valvifer: shape
(0) elongated
Ventral margin of the second valvifer: curvature
(0) curved medially and curved dorsally in lateral view
Ventral margin of the second valvifer: position
(0) surrounds posterior second valvifer-second valvula muscle
Venom gland reservoir of the second valvifer: count
(0) present
(1) absent
Venom gland reservoir of the second valvifer: physical quality
(0) resinous
Distal region of first valvula: shape
(0) tapered
(1) spatulate
Banding pattern on first valvula: count
(0) absent
Annuli on first valvula: count
(0) absent
Processus articularis: position
(0) anterolateral region of the bulb
Anterior notch of the dorsal valve: position
(0) anterior region of the bulb
Processus musculares: position
(0) anterodorsal region of the bulb
Distal notch of the dorsal valve: count
(0) absent
Distal region of the dorsal valve: shape
(0) tapered dorsal view
Anterior region of dorsal margin of the bulb: curvature
(0) curved dorsally in lateral view
Bulb: height in lateral view vs. height of anterior area of the second valvifer in lateral view
(0) =0.5
(1) <0.5
Banding pattern on dorsal valve: count
(0) absent
Annuli on dorsal valve: count
(0) present
(1) absent
Annuli on dorsal valve: count value
(0) four
(1) six
(0) present
Distal vertical conjunctiva of the second valvifer-third valvifer complex: count
(0) present
Distal vertical conjunctiva of the second valvifer-third valvifer complex: overlap relationship with dorsal margin of second valvifer-third valvula complex
(0) does not overlap
Distal region of the third valvula: shape
Lateral region of the third valvula: shape
(0) convex in posterior view
Lateral region of the third valvula: entity
(0) is_a sclerite
Medial region of the third valvula: shape
(0) concave in posterior view
Median region of the third valvula: entity
(0) is_a conjunctiva
(0) quadrangular in lateral view
Dorso-median region of T9: width in dorsal view vs. height in dorsal view
(0) present
Anterolateral region of anterior ridge of T9: position
(0) adjacent to anterior margin of T9:
Distance between Anterior ridge of T9 and anterior margin of T9: anterior-posterior gradient
(0) increasing
Attachment site of S7-first valvula muscle (M1) on S7: position
(0) adjacent to the anterior margin of S7
Attachment site of S7-first valvula muscle (M1) on first valvula: position
Attachment site of S7-first valvula muscle on first valvula: position
(0) anterior to attachment site of S7-first valvula muscle on S7
Attachment site of dorsal T8-T9 muscle on T8: position
(0) adjacent to the anterior margin of T8
Attachment site of dorsal T8-T9 muscle on T9: position
(0) adjacent to anterior margin of the dorsomedial region of T9
Attachment site of lateral T8-T9 muscle on T8: position
(0) posterodorsal to the attachment site of dorsal T8-T9 muscle on T8
Attachment site of lateral T8-T9 muscle on T9: position
(0) adjacent to anterior ridge of T9
Attachment site of T8-first valvifer muscle on T8: position
(0) dorsal to the attachment site of lateral T8-T9 muscle on T8
Attachment site of T8-first valvifer muscle on first valvifer: position
(0) adjacent to the ninth tergal condyle of the first valvifer
Attachment site of T8-first valvifer muscle on first valvifer: position
(0) on ventral sclerite of the first valvifer adjacent to the intravalvifer articulation
Attachment site of dorsal T9-second valvifer muscle on T9: position
(0) adjacent to the anterior ridge of T9 and the region of T9 dorsal to and ventral to the anterior ridge of T9
Attachment site of dorsal T9-second valvifer muscle on second valvifer: position
(0) adjacent to the anterior section of the dorsal flange of the second valvifer
Ventral T9-second valvifer muscle: count value
(0) 1
Attachment site of ventral T9-second valvifer muscle on second valvifer: position
(0) posterior area of the second valvifer
Attachment site of ventral T9-second valvifer muscle on T9: position
(0) adjacent to anterior region of anterior ridge of T9
First valvifer-second valvifer muscle: count
(0) present
(1) absent
Attachment site of posterior T9-second valvifer muscle on T9: position
(0) on the dorsal region of T9
Attachment site of posterior T9-second valvifer muscle on second valvifer: position
(0) on the median bridge
First valvifer-genital membrane muscle: shape
(0) fan shaped in dorsal view
Attachment site of first valvifer-genital membrane muscle on first valvifer: position
(0) on the medial side of the first valvifer adjacent to the intervalvifer articulation
Attachment site of first valvifer-genital membrane muscle on genital membrane: position
(0) along the median line of the genital membrane
Attachment site of first valvifer-genital membrane muscle on first valvifer: position
(0) on the medial side of the dorsal sclerite of the first valvifer
Attachment site of posterior second valvifer-second valvula muscle on second valvifer: position
(0) on the medial side of the posterior area of the second valvifer
(1) on the medial side of the posterior area of the second valvifer and the anterior area of the second valvifer
Attachment site of posterior second valvifer-second valvula muscle on second valvula: position
(0) on the processus musculares
T9-genital membrane muscle: count
(0) absent
Lateral T9-second valvifer muscle: count
(0) absent
second valvifer-genital membrane muscle: count
(0) absent
anterior second valvifer-second valvula muscles: count
(0) absent
The enormous diversity of ovipositor phenotype in Hymenoptera reflects the manner in which the female wasp finds feasible environments for her developing larvae. Host structure and location, as well as the different ways of storing the ovipositor, are arguably the principal factors driving the structural adaptation of these structures (
The ceraphronoid ovipositor is stored in a horizontal position inside the metasoma, with its ventral part concealed by S7 (Fig. 6E). As the first oviposition movement, the contracting muscles between the apical metasomal tergites and sternites expose the ventral part of the ovipositor by rotating it and the ninth abdominal tergite posteriorly, from the resting, horizontal to the active, vertical position. This movement is common within Apocrita (Fig. 6F;
The mechanism of the extension of the terebra from the second valvifer-third valvula complex is shared between Ceraphronoidea and most other Hymenoptera. The movement is facilitated by the posterior second valvifer-second valvula muscles (M9: Figs 1A–F, 2A, B, D, 3A, B, 4B, D–F, 5A, C, D;
At the end of the oviposition the terebra is retracted prior to the anterior rotation of the ovipositor/T9 complex into the resting, horizontal position. The retraction of the terebra, unlike its extension, seems to be unique for Ceraphronoidea. In other Hymenoptera the movement is accomplished by the contraction of the vertically oriented anterior second valvifer-second valvula muscle that arises from the anterodorsal margin of the second valvifer and inserts on the distal region of the bulb (
The paired first and second valvifers and T9, operated together by the dorsal and ventral T9-second valvifer muscles, form the ovipositor machinery that is responsible for “drilling” the terebra into a substrate and moving the egg along the egg canal (
As the dorsal T9-second valvifer muscle (M5: Figs 1A, B, D, E, 2A, B, D, 3B, 4A, B, D, E, F, 5A–C, 6A) contracts the first valvifer pivots posteriorly (in anterior to the left position) at the intervalvifer articulation while contraction of the antagonistic ventral T9-second valvifer muscle (M6: Figs 1A, B, D, E, 2A, B, D, 3B, 4A, B, D, F, 5A, B, 6A) pulls the first valvifer in the opposite direction (compare the position of the tergo-valvifer (tva) and intervalvifer articulations (iva) on Figures 3A and 3E). As this movement is what slides the first valvula along the second valvulae, the distance the first valvifer moves determines the distance the first valvula moves. The left and right first valvulae slide back and forth alternately during the alternate contraction of the left and right T9-second valvifer muscle pairs (1vf left, 1vv right: Fig. 3B;
Adaptations effecting the alternate movements and configuration of the first valvulae might be mostly affected by the hardness of the substrate and constraints for fast oviposition. Oviposition into a concealed, and therefore relatively immobile host requires a robust system that has to be strong enough to drill or break the barrier. On the other hand, parasitization of an exposed, mobile but relatively soft host requires fast and perhaps less robust mechanism. Two major egg laying habits have been recorded within Ceraphronoidea: oviposition inside a mobile host and oviposition trough a hard but relatively thin barrier enclosing the host, which has restricted movement (
The relative distance between the anterior angle of the first valvifer and the intervalvifer articulation (ang, iva: Figs 1B, 2C, E, 3C, E, 4A, E, F, 5A, 6A, C, D) is most probably positively correlated with the degree of the sliding motion of the first valvula (Prentice 1998). The posterior margin of the first valvifer angled at the tergo-valvifer articulation (tva: Fig.) in most Hymenoptera (
Two ridges/apodemes are present on T9 in most Hymenoptera, the anterior flange of T9 and the cordate apodeme. The anterior flange extends along the anterior margin of the tergite and might be homologous with the antecosta of the ninth abdominal tergum of other insects because it receives the site of attachment of the dorsal T8-T9 muscle in Macroxyela (
The first valvifer is composed of two articulating sclerites in Trassedia. Although this condition is possibly plesiomorphic for Insecta (
The presence or absence of annuli at the tip of the ovipositor may depend on the hardness of the substrate into which the wasp is ovipositing, as well as the circumstances under which oviposition is taking place (
A minute gland (dgl?: Figs 3B, 5A) and a relatively larger gland reservoir (res: Figs 3A–E, 5A, B, E, F, 4C), enclosed by the second valvifers, have been detected in Ceraphronidae including Trassedia and Lagynodes. The Dufour’s gland and the venom gland reservoir has a similar location in some Chalcidoidea (
In general, the ovipositors of Ceraphron, Trassedia and Aphanogmus sp. 2 are less robust and capable of a very large degree of motion, corresponding to the available data about ovipositing in exposed and active hosts. Trassedia represents, perhaps, a more extreme version of the “quickly into soft substrate” oviposition type. Megaspilidae, on the other hand, have a stronger, more robust ovipositor systems, which afford the smaller degree of motion required for handling a harder substrate concealing a static host. Dendrocerus, for example, exhibits extended sites of origins for muscles and a very small degree of motion for the first valvulae. Aphanogmus sp. 1, although it belongs to Ceraphronidae, shares numerous characteristics with Dendrocerus and therefore may represent the Aphanogmus-group that parasitizes hosts obscured by harder barrier, e.g., the wall of a plant gall, and thus is a case of parallelism driven by the same environmental constraints.
So far it is widely accepted that Ceraphronoidea is composed of two extant families, Ceraphronidae and Megaspilidae, plus two fossil families not treated here. The limits between the two families, however, have been challenged recently (
We thank the following curators who loaned material to conduct this study: Lubomír Masner from the Canadian National Collection, Brian Fisher from California Academy of Sciences, Bob Zuparko from California Academy of Sciences and the Berkeley Essig Museum, Michael Sharkey from the University of Kentucky, and Dave Karlsson of Uppsala University. We are grateful to Matt Yoder, Lars Vilhelmsen, Katja Seltmann, Matt Bertone, Patricia Mullins, Heather Campbell, Bob Blinn, Lubomír Masner and Jim Balhoff for valuable input and assistance with this manuscript. We especially thank Eva Johannes (Cellular and Molecular Imaging Facility, NCSU) and Missy Hazen (Penn State Microscopy and Cytometry Facility - University Park, PA) for her help with CLSM, Chuck Mooney (Analytical Instrumentation Facility, NCSU) for his assistance with SEM and Rolf Beutel. This research was funded in part by the U. S. National Science Foundation (grants DBI-0850223, DEB-0842289) and benefited from discussions initiated through the Phenotype Research Coordination Network (NSF DEB-0956049).
Anatomical terms used, cross-referenced to an ontological (formal) definition (Hymenoptera Anatomy Ontology; URI = Uniform Resource Identifier).
| Abbreviation | Label | Concept | URI |
|---|---|---|---|
| absent | A quality denoting the lack of an entity. | http://purl.obolibrary.org/obo/PATO_0000462 | |
| adjacent to | A spatial quality inhering in a bearer by virtue of the bearer’s being located near in space in relation to another entity. | http://purl.obolibrary.org/obo/PATO_0002259 | |
| ann | annulus, annuli | The carina that is transverse and extends across the lateral wall of the terebra. | http://purl.obolibrary.org/obo/HAO_0001173 |
| ang | anterior angle of the first valvifer | The corner on the first valvifer that marks the posterior end of the first valvula. | http://purl.obolibrary.org/obo/HAO_0002168 |
| aa | anterior area of the second valvifer | The area of the second valvifer which is anterior to the anatomical line that is the shortest distance from the first valviferal fossa of the second valvifer and the ventral margin of the second valvifer | http://purl.obolibrary.org/obo/HAO_0002169 |
| anterior flange of T9 | The flange that extends along the anterolateral margin of female T9. | http://purl.obolibrary.org/obo/HAO_0001171 | |
| af1 | anterior flange of the first valvifer | The flange that extends anteriorly on the first valvifer and overlaps with the posterior margin of the anterior area of the second valvifer. | http://purl.obolibrary.org/obo/HAO_0002166 |
| anterior margin | anatomical margin and (overlaps some anterior side) | http://purl.obolibrary.org/obo/BSPO_0000671 | |
| an2 | anterior notch of the dorsal valve | The notch that is located anteriorly on the dorsal ramus of the second valvula that accommodates the ventral ramus of the second valvula and the first valvula. | http://purl.obolibrary.org/obo/HAO_0002178 |
| anterior region, anteriorly | anatomical region and (overlaps some anterior side) | http://purl.obolibrary.org/obo/BSPO_0000071 | |
| ar9 | anterior ridge of T9 | The ridge that extends along the anterior margin of female T9 and receives the site of origin of the ventral and the dorsal T9-second valvifer muscles. | http://purl.obolibrary.org/obo/HAO_0002182 |
| anterior second valvifer-second valvula muscle | The ovipositor muscle that arises from the anterodorsal part of the second valvifer and inserts subapically on the processus articulares. | http://purl.obolibrary.org/obo/HAO_0001166 | |
| asf | anterior section of dorsal flange of the second valvifer | The area of the dorsal flange of the second valvifer that is anterior to the site of origin of the basal line. | http://purl.obolibrary.org/obo/HAO_0002173 |
| anterior to | A spatial quality inhering in a bearer by virtue of the bearer’s being located toward the front of an organism relative to another entity. | http://purl.obolibrary.org/obo/PATO_0001632 | |
| anterodorsal margin | anatomical margin and (overlaps some anterior side) and (overlaps some dorsal side) | http://purl.obolibrary.org/obo/BSPO_0000686 | |
| anterolateral region | Anatomical region and (overlaps some anterior side) and (overlaps some lateral side) | http://purl.obolibrary.org/obo/BSPO_0000029 | |
| apodeme | The process that is internal. | http://purl.obolibrary.org/obo/HAO_0000142 | |
| attachement site | The area of the integument where muscles are attached to epidermal cells. | http://purl.obolibrary.org/obo/HAO_0002184 | |
| banding pattern | The anatomical cluster that is composed of the strongly sclerotised areas corresponding with the annuli and less strongly sclerotised areas situated between them. | http://purl.obolibrary.org/obo/HAO_0001176 | |
| basal articulation | The articulation that is part of the second valvifer-second valvula-third valvula complex and adjacent to the rhachis. | http://purl.obolibrary.org/obo/HAO_0001177 | |
| bl | basal line of the second valvifer | The line on the second valvifer that extends between the pars articularis and the dorsal flange of second valvifer. | http://purl.obolibrary.org/obo/HAO_0002171 |
| bent | A shape quality inhering in a bearer by virtue of the bearer’s having one or more angle(s) in its length. | http://purl.obolibrary.org/obo/PATO_0000617 | |
| blunt | A shape quality inhering in a bearer by virtue of the bearer’s terminating gradually in a rounded end. | http://purl.obolibrary.org/obo/PATO_0001950 | |
| bulb | bulb | The anterior area of the dorsal valve that is bulbous. | http://purl.obolibrary.org/obo/HAO_0002177 |
| concave | A shape quality in a bearer by virtue of the bearer’s curving inward. | http://purl.obolibrary.org/obo/PATO_0001857 | |
| conjunctiva | The area of the integument that is weakly sclerotized, with thin exocuticle. | http://purl.obolibrary.org/obo/HAO_0000221 | |
| convex | A shape quality that obtains by virtue of the bearer having inward facing edges; having a surface or boundary that curves or bulges outward, as the exterior of a sphere. | http://purl.obolibrary.org/obo/PATO_0001355 | |
| cordate apodeme | The apodeme on the anterior margin of the abdominal tergum 9 that receives the ventral T9-second valvifer muscle. | http://purl.obolibrary.org/obo/HAO_0001585 | |
| corner | The projection that is located at the intersection of two or more edges. | http://purl.obolibrary.org/obo/HAO_0000223 | |
| count | The number of entities of this type that are part of the whole organism. | http://purl.obolibrary.org/obo/PATO_0000070 | |
| count value | http://purl.obolibrary.org/obo/PATO_0000416 | ||
| curvature | A surface shape quality inhering in a bearer by virtue of the bearer’s exhibiting a degree of bending. | http://purl.obolibrary.org/obo/PATO_0001591 | |
| curved dorsally | A curvature quality inhering in a bearer by virtue of the bearer’s being curved towards the back or upper surface of an organism. | http://purl.obolibrary.org/obo/PATO_0001468 | |
| curved medially | A curvature quality inhering in a bearer by virtue of the bearer’s being curved towards the middle. | http://purl.obolibrary.org/obo/PATO_0002164 | |
| distal notch of the dorsal valve | The notch that is distal on the dorsal valve. | http://purl.obolibrary.org/obo/HAO_0002179 | |
| distal region | Anatomical region and (overlaps some distal side) | http://purl.obolibrary.org/obo/BSPO_0000078 | |
| dvc | distal vertical conjunctiva of the second valvifer-third valvifer complex | The conjunctiva that traverses the second valvifer-third valvula complex and is located distal to the median bridge of the second valvifer. | http://purl.obolibrary.org/obo/HAO_0002180 |
| distance | A quality that is the extent of space between two entities. | http://purl.obolibrary.org/obo/PATO_0000040 | |
| dorsal margin | Anatomical margin and (overlaps some dorsal side). | http://purl.obolibrary.org/obo/BSPO_0000679 | |
| dp | dorsal projection of the second valvifer | The projection that is located on the second valvifer and corresponds to the proximal end of the rachis. | http://purl.obolibrary.org/obo/HAO_0002172 |
| dorsal region | anatomical region and (overlaps some dorsal side) | http://purl.obolibrary.org/obo/BSPO_0000079 | |
| d1vf | dorsal sclerite of the first valvifer | The sclerite of the first valvifer that is located dorsally of the transvalviferal conjunctiva. | http://purl.obolibrary.org/obo/HAO_0002163 |
| M2 | dorsal T8-T9 muscle | The abdominal muscle that arises from the anteromedian margin of female T8 and inserts on the anteromedian margin of the female T9. | http://purl.obolibrary.org/obo/HAO_0001571 |
| M5 | dorsal T9-second valvifer muscle | The ovipositor muscle that arises along the posterodorsal part of the anterior margin of female T9 and inserts on the anterior section of the dorsal flanges of the second valvifer. | http://purl.obolibrary.org/obo/HAO_0001569 |
| dorsal to | x dorsal_to y if x is further along the dorso-ventral axis than y, towards the back. A dorso-ventral axis is an axis that bisects an organism from back (e.g. spinal column) to front (e.g. belly). | http://purl.obolibrary.org/obo/BSPO_0000098 | |
| dorsal valve | The area that is articulated with the right and left second valvifers at the basal articulation and bears the rhachies. | http://purl.obolibrary.org/obo/HAO_0001658 | |
| dorsal view | http://purl.obolibrary.org/obo/BSPO_0000063 | ||
| dorsomedial region | anatomical region and (overlaps some dorsal side) and (overlaps some medial side) | http://purl.obolibrary.org/obo/BSPO_0000069 | |
| ec | egg canal | The anatomical space that is between the left and right rhachises. | http://purl.obolibrary.org/obo/HAO_0002191 |
| elongated | A quality inhering in a bearer by virtue of the bearer’s length being notably higher than its width. | http://purl.obolibrary.org/obo/PATO_0001154 | |
| fan-shaped | A quality inhering in a bearer that is shaped in the form of a fan. | http://purl.obolibrary.org/obo/PATO_0002219 | |
| 1vf | first valvifer | The area of the first valvifer-first valvula complex that is proximal to the aulax, bears the ninth tergal condyle of the first valvifer and the second valviferal condyle of the first valvifer and is connected to the genital membrane by muscle. | http://purl.obolibrary.org/obo/HAO_0000338 |
| M8 | first valvifer-genital membrane muscle | The ovipositor muscle that arises from the posterior part of the first valvifer and inserts anteriorly on the genital membrane anetrior to the T9-genital membrane muscle. | http://purl.obolibrary.org/obo/HAO_0001746 |
| first valvifer-second valvifer muscle | The ovipositor muscle that arises from the first valvifer and inserts on the second valvifer. | http://purl.obolibrary.org/obo/HAO_0002189 | |
| 1vv | first valvula, first valvulae | The area of the first valvifer-first valvula complex that is delimited distally by the proximal margin of the aulax. | http://purl.obolibrary.org/obo/HAO_0000339 |
| gm | genital membrane | The conjunctiva that connects the ventral margins of the second valvifers. | http://purl.obolibrary.org/obo/HAO_0001757 |
| height | A 1-D extent quality inhering in a bearer by virtue of the bearer’s vertical dimension of extension. | http://purl.obolibrary.org/obo/PATO_0000119 | |
| increasing | quality and (increased_in_magnitude_relative_to some normal) | http://purl.obolibrary.org/obo/PATO_0002300 | |
| iar | interarticular ridge of the first valvifer | The ridge that extends along the posterior margin of the first valvifer between the intervalvifer and tergovalvifer articulations. | http://purl.obolibrary.org/obo/HAO_0001562 |
| iva | intervalvifer articulation | The articulation between the first valvifer and second valvifer. | http://purl.obolibrary.org/obo/HAO_0001558 |
| iava | intravalvifer articulation | The articulation between the dorsal sclerite of the first valvifer and the ventral sclerite of the first valvifer. | http://purl.obolibrary.org/obo/HAO_0002165 |
| lateral region | anatomical region and (overlaps some lateral side) | http://purl.obolibrary.org/obo/BSPO_0000082 | |
| M3 | lateral T8-T9 muscle | The ninth abdominal tergal muscle that arises from the anterolateral margin of female T8 and inserts on the anterolateral margin of female T9. | http://purl.obolibrary.org/obo/HAO_0001776 |
| lateral T9-second valvifer muscle | The muscle that arises from the posteroventral parts of the female T9 and inserts on the median bridge. | http://purl.obolibrary.org/obo/HAO_0002187 | |
| lateral view | http://purl.obolibrary.org/obo/BSPO_0000066 | ||
| length of anterior area of second valvifer | The anatomical line that is parallel with the longitudinal body axis and the shortest among the anatomical lines that extends between the anterior and posterior margins of the anterior area of the second valvifer. | http://purl.obolibrary.org/obo/HAO_0002240 | |
| length of dorsal projection | The anatomical line that is parallel with the longitudinal body axis and the shortest among the anatomical lines that extends between the anterior and posterior margins of the dorsal projection. | http://purl.obolibrary.org/obo/HAO_0002193 | |
| length of female T9 | The anatomical line that is parallel with the longitudinal body axis and the shortest among the anatomical lines that extend between the anterior and posterior margins of female T9. | http://purl.obolibrary.org/obo/HAO_0002241 | |
| medial side | a point in the centre of the organism (where the left-right axis intersects the midsagittal plane) | http://purl.obolibrary.org/obo/BSPO_0000067 | |
| median bridge | The area that connects posterodorsally the second valvifers and is the site of attachment for the posterior T9-second valvifer muscle. | http://purl.obolibrary.org/obo/HAO_0001780 | |
| mc2 | distal notch of the dorsal valve | The notch that is distal on the dorsal valve. | http://purl.obolibrary.org/obo/HAO_0002179 |
| mc1 | medial conjunctiva of the first valvulae | The conjunctiva that extends medially along the first valvula. | http://purl.obolibrary.org/obo/HAO_0002192 |
| median line | An axis that bisects an organism from head end to opposite end of body or tail. | http://purl.obolibrary.org/obo/BSPO_0000013 | |
| medial region | anatomical region and (overlaps some medial side) | http://purl.obolibrary.org/obo/BSPO_0000083 | |
| ninth tergal condyle of the first valvifer | The condyle that is located on the first valvifer and articulates with the first valviferal fossa of T9. | http://purl.obolibrary.org/obo/HAO_0002160 | |
| oth | olistheters | The anatomical cluster that is composed of the rhachis of the second valvula and the aulax of the first valvula. | http://purl.obolibrary.org/obo/HAO_0001103 |
| overlaps | x overlaps y if they have some part in common. | http://purl.obolibrary.org/obo/bspo%23overlaps | |
| ovipositor | The anatomical cluster that is composed of the first valvulae, second valvulae, third valvulae, first valvifers, second valvifers and female T9. | http://purl.obolibrary.org/obo/HAO_0000679 | |
| paa | pars articularis | The articular surface that is situated anteriorly on the ventral margin of the second valvifer and forms the lateral part of the basal articulation. | http://purl.obolibrary.org/obo/HAO_0001606 |
| position | A spatial quality inhering in a bearer by virtue of the bearer’s spatial location relative to other objects in the vicinity. | http://purl.obolibrary.org/obo/PATO_0000140 | |
| posteriorly | anatomical gradient and (has_axis some anterior/posterior axis) | http://purl.obolibrary.org/obo/BSPO_0000052 | |
| pa | posterior area of the second valvifer | The area of the second valvifer that is posterior to the anatomical line that is the shortest distance from the first valviferal fossa of the second valvifer to the ventral margin of the second valvifer. | http://purl.obolibrary.org/obo/HAO_0002170 |
| posterior margin | anatomical margin and (overlaps some posterior side) | http://purl.obolibrary.org/obo/BSPO_0000672 | |
| p1v | posterior margin of first valvifer | The margin of the first valvifer that is posterior and extends between the intervalvifer articulation and the anterior angle of the first valvifer. | http://purl.obolibrary.org/obo/HAO_0002159 |
| M9 | posterior second valvifer-second valvula muscle | The ovipositor muscle that arises posteroventrally from the second valvifer and inserts on the processus musculares of the second valvula. | http://purl.obolibrary.org/obo/HAO_0001815 |
| psf | posterior section of dorsal flange of the second valvifer | The area of the dorsal flange of the second valvifer that is posterior to the to the site of origin of the basal line. | http://purl.obolibrary.org/obo/HAO_0002174 |
| M7 | posterior T9-second valvifer muscle | The ovipositor muscle that arises medially from the posterodorsal part of female T9 and inserts on the median bridge of the second valvifers. | http://purl.obolibrary.org/obo/HAO_0001813 |
| posterior view | http://purl.obolibrary.org/obo/BSPO_0000056 | ||
| postero-medial region | anatomical region and (overlaps some posterior side) and (overlaps some medial side) | http://purl.obolibrary.org/obo/BSPO_0000070 | |
| posterodorsal to | A spatial quality inhering in a bearer by virtue of the bearer’s being located toward the rear and upper surface of an organism relative to another entity. | http://purl.obolibrary.org/obo/PATO_0001916 | |
| posteroventral corner of first valvifer | The corner of the first valvifer that is adjacent to the intervalvifer articulation. | http://purl.obolibrary.org/obo/HAO_0002239 | |
| present | A quality inhering in a bearer by virtue of the bearer’s existence. | http://purl.obolibrary.org/obo/PATO_0000467 | |
| pra | processus articularis | The process that extends laterally from the proximal region of the second valvula and forms the median part of the basal articulation, and corresponds to the site of attachment for the anterior second valvifer-second valvula muscle. | http://purl.obolibrary.org/obo/HAO_0001704 |
| prm | processus musculares | The apodeme that extends dorsally from the proximal part of the second valvula to the genital membrane and receives the site of attachment of the posterior second valvifer-second valvula muscle. | http://purl.obolibrary.org/obo/HAO_0001703 |
| proportion | A quality inhering in a bearer by virtue of the bearer’s magnitude in respect to a related entity. | http://purl.obolibrary.org/obo/PATO_0001470 | |
| quadrangular | A shape quality inhering in a bearer by virtue of the bearer’s having four angles and four sides. | http://purl.obolibrary.org/obo/PATO_0001988 | |
| region | A 3D region in space without well-defined compartmental boundaries; for example, the dorsal region of an ectoderm. | http://purl.obolibrary.org/obo/BSPO_0000070 | |
| resinous | A physical quality inhering in a bearer by virtue of the bearer exhibiting molecular attraction to another entity in contact. | http://purl.obolibrary.org/obo/PATO_0002331 | |
| ridge | The apodeme that is elongate. | http://purl.obolibrary.org/obo/HAO_0000899 | |
| S7 | The sternite that is connected to the first valvula via muscles. | http://purl.obolibrary.org/obo/HAO_0002185 | |
| S7-first valvula muscle | The muscle that originates from the abdominal sternum 7 and inserts on the first valvula. | http://purl.obolibrary.org/obo/HAO_0001668 | |
| sclerite, sclerites | The area of the integument where the cuticle is well sclerotised with thick exocuticle. | http://purl.obolibrary.org/obo/HAO_0000909 | |
| second valvifer-genital membrane muscle | The ovipositor muscle that arises anteriorly from the dorsal flange of the second valvifer and inserts anteriorly on the dorsal part of the genital membrane. | http://purl.obolibrary.org/obo/HAO_0001672 | |
| 2vf | second valvifer | The area of the second valvifer-second valvula-third valvula complex that is proximal to the basal articulation and to the processus musculares and articulates with female T9. | http://purl.obolibrary.org/obo/HAO_0000927 |
| second valvifer-genital membrane muscle | The ovipositor muscle that arises anteriorly from the dorsal flange of the second valvifer and inserts anteriorly on the dorsal part of the genital membrane. | http://purl.obolibrary.org/obo/HAO_0001672 | |
| second valviferal condyle of the first valvifer | The condyle that is located on the first valvifer and articulates with the first valviferal fossa of the second valvifer. | http://purl.obolibrary.org/obo/HAO_0002167 | |
| second valvifer-third valvula complex | The area of the second valvifer-second valvula-third valvula complex that is proximal to the basal articulation. | http://purl.obolibrary.org/obo/HAO_0002181 | |
| 2vv | second valvula, second valvulae | The area of the second valvifer-second valvula-third valvifer complex that is distal to the basal articulation and to the processus musculares and is limited medially by the median body axis. | http://purl.obolibrary.org/obo/HAO_0000928 |
| spa | sensillar patch | The patch that is composed of placoid sensilla adjacent to the intervalvifer articulation. | http://purl.obolibrary.org/obo/HAO_0001671 |
| shape | A morphological quality inhering in a bearer by virtue of the bearer’s ratios of distances between its features (points, edges, surfaces and also holes etc). | http://purl.obolibrary.org/obo/PATO_0000052 | |
| sharpness | A shape quality inhering in a bearer by virtue of the bearer’s having a sharp or tapered end or point. | http://purl.obolibrary.org/obo/PATO_0000944 | |
| sharp | A shape quality inhering in a bearer by virtue of the bearer’s terminating in a point or edge. | http://purl.obolibrary.org/obo/PATO_0001419 | |
| spatulate | A shape quality inhering in a bearer by virtue of the bearer’s being oblong, with the lower end very much attenuated. | http://purl.obolibrary.org/obo/PATO_0001937 | |
| straight | A shape quality inhering in a bearer by virtue of the bearer’s being free of curves, bends, or angles. | http://purl.obolibrary.org/obo/PATO_0002180 | |
| surrounds | http://purl.obolibrary.org/obo/BSPO_0000101 | ||
| T8 | The tergite that is connected to female T9 by muscles. | http://purl.obolibrary.org/obo/HAO_0002188 | |
| T8-first valvifer muscle | The ovipositor muscle that originates from the lateral part of female T8 and inserts on the dorsal margin of the first valvifer. | http://purl.obolibrary.org/obo/HAO_0001640 | |
| T9 | The tergite that is articulted with the first valvifer and second valvifer and is connected to the second valvifer via muscles. | http://purl.obolibrary.org/obo/HAO_0000075 | |
| T9-genital membrane muscle | The ovipositor muscle that arises from the cordate apodeme and inserts dorsally on the proximal part of the genital membrane and on the opposite cordate apodeme. | http://purl.obolibrary.org/obo/HAO_0001639 | |
| tapered | A shape quality inhering in a bearer by virtue of the bearer’s being gradually narrower or thinner toward one end. | http://purl.obolibrary.org/obo/PATO_0001500 | |
| trb | terebra | The anatomical cluster that is composed of the first and second valvulae. | http://purl.obolibrary.org/obo/HAO_0001004 |
| tva | tergo-valvifer articulation | The articulation that is located between the abdominal tergum 9 and the first valvifer and is composed of the ninth tergal condyle of the first valvifer and the first valviferal fossa of the ninth tergite. | http://purl.obolibrary.org/obo/HAO_0001636 |
| thickened | A thickness which is relatively high. | http://purl.obolibrary.org/obo/PATO_0000591 | |
| thickness | A 1-D extent quality which is equal to the dimension through an object as opposed to its length or width. | http://purl.obolibrary.org/obo/PATO_0000915 | |
| 3vv | third valvula, third valvulae | The area of the second valvifer-third valvula complex that is posterior to the distal vertical conjunctiva of the second valvifer-third valvula complex. | http://purl.obolibrary.org/obo/HAO_0001012 |
| tvc | transvalvifer conjunctiva | The conjunctiva that traverses the first valvifer and separates the dorsal and ventral sclerites of the first valvifer. | http://purl.obolibrary.org/obo/HAO_0002162 |
| triangular | A shape quality inhering in a bearer by virtue of the bearer’s having three angles. | http://purl.obolibrary.org/obo/PATO_0001875 | |
| res | venom gland reservoir of the second valvifer | The gland reservoir that is between the second valvifers. | http://purl.obolibrary.org/obo/HAO_0002176 |
| ventral margin | anatomical margin and (overlaps some ventral side) | http://purl.obolibrary.org/obo/BSPO_0000684 | |
| vr2 | ventral ramus of the second valvula | The area of the second valvifer-second vavula-third valvifer complex that bears the rhachis. | http://purl.obolibrary.org/obo/HAO_0001107 |
| v1v | ventral sclerite of the first valvifer | The sclerite of the first valvifer that is ventral to the transvalviferal conjunctiva. | http://purl.obolibrary.org/obo/HAO_0002164 |
| M6 | ventral T9-second valvifer muscle | The ovipositor muscle that arises from the lateral region of female T9 and inserts along the posterior part of the dorsal flange of the second valvifer. | http://purl.obolibrary.org/obo/HAO_0001616 |
| ventral to | x ventral_to y if x is further along the dorso-ventral axis than y, towards the front. A dorso-ventral axis is an axis that bisects an organism from back (e.g. spinal column) to front (e.g. belly). | http://purl.obolibrary.org/obo/BSPO_0000102 | |
| width | A 1-D extent quality which is equal to the distance from one side of an object to another side which is opposite. | http://purl.obolibrary.org/obo/PATO_0000921 |