(C) 2013 John S. LaPolla. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: LaPolla JS, Hawkes PG, Fisher JN (2013) Taxonomic review of the ant genus Paratrechina, with a description of a new species from Africa. Journal of Hymenoptera Research 35: 71–82. doi: 10.3897/JHR.35.5628
With the recent finding of Paratrechina (broad sense) paraphyly, only Paratrechina longicornis remained in a redefined genus. As one of the most widely distributed ant species due to human transfer around the world, there is much interest in the biology of P. longicornis. One issue concerning P. longicornis has been as to where exactly the species is native, with both African and Asian native ranges being invoked in the literature. Here we report the discovery of a second species within Paratrechina. This species, P. zanjensis, is native to Africa (known from Angola, Mozambique and Tanzania), where it appears to be a dry miombo woodland species. Given the discovery of this new species, a reevaluation of the morphological definition of Paratrechina is provided; also provided is an updated generic level identification key. Given the available distribution information on P. longicornis, we conclude that P. longicornis remains most likely native to southeastern Asia, and that the discovery of a new species native to Africa makes Paratrechina yet another example of an ant genus that possesses an Afro-Asian distribution.
Invasive species, miombo woodland, new species, Nylanderia, Prenolepis, Zatania
Recently the ant genus Paratrechina (broad sense) was found to be paraphyletic, with all but one species being transferred to the revived genera Nylanderia and Paraparatrechina (
One particularly interesting and important aspect of Paratrechina longicornis biology is that it is perhaps the most widespread ant species in the world (
The unusual morphology of Paratrechina longicornis, coupled with its widespread tramp status, makes the discovery of a new species within the genus particularly important. Here we report on such a discovery from Africa (records from Angola, Mozambique and Tanzania). The discovery of this new species of Paratrechina now requires a reevaluation of the previous morphological definition of the genus (
Specimens examined for this study are deposited in the following institutions:
AFRC AfriBugs, Pretoria, South Africa
ANIC Australian National Insect Collection, Canberra, Australia
CASC California Academy of Sciences, San Francisco, CA, USA
MCZC Museum of Comparative Zoology, Cambridge, MA, USA
NMKE National Museum of Kenya, Nairobi, Kenya
SAMC Iziko South African Museum, Cape Town, South Africa
USNM National Museum of Natural History, Washington, DC, USA
Measurements were taken using an eyepiece graticule with a Leica MZ16 microscope mounted on an axial shift carrier to avoid parallax errors, recorded to the nearest 1/10 graticule unit and lengths calculated using calibration data from a stage micrometer for each magnification. All measurements are given in millimeters. Digital color images of a Paratrechina longicornis male were created using a JVC KY-F75 digital camera and Syncroscopy Auto-Montage (v 5.0) software; color images of Paratrechina zanjensis and Paratrechina longicornis workers were created using a Leica DFC425 digital camera and Leica LAS Montage (v3.8) software. Morphological terminology for measurements and indices employed throughout are defined (following
EL (Eye Length): maximum length of compound eye in full-face view
GL (Gaster Length): the length of the gaster in lateral view from the anteriormost point of the first gastral segment (third abdominal segment) to the posteriormost point
HL (Head Length): the length of the head proper, excluding the mandibles; measured in full-face view from the midpoint of the anterior clypeal margin (a line is drawn between the projecting lateral portions of the clypeus to accommodate the anterior margin which is medially indented in Paratrechina) to a line drawn across the posterior margin from its highest points
HW (Head Width): the maximum width of the head in full-face view, excluding eyes
MSC (Mesonotal Setal Count): the number of erect macrosetae on mesonotum to one side of the sagittal plane
PW (Pronotal Width): the maximum width of the pronotum in dorsal view
PrFL (Profemur Length): the length of the profemur from its margin with the trochanter to its margin with the tibia
PSC (Pronotal Setal Count): the number of erect macrosetae on pronotum to one side of the sagittal plane
SSC (Scape Setal Count): the number of erect macrosetae on one antennal scape, excluding the terminal setal cluster
SL (Scape Length): the maximum length of the antennal scape excluding the condylar bulb
TL (Total Length): HL+WL+GL
WL (Weber’s Length): in lateral view, the distance from the posteriormost border of the metapleural lobe to the anteriormost border of the pronotum, excluding the neck
CI (Cephalic Index): (HW/HL) • 100
REL2 (sensu
Ward 2006 ): (EL/HW) • 100SI (Scape Index): (SL/HW) • 100
Since the new species described here is only known from the worker, we provide only a worker-based definition for the genus pending the discovery of males and queens for Paratrechina zanjensis.
Monomorphic, medium sized (2.1–3.2 mm in total length) formicine ants; brown to dark brown in color, with lighter mandibles, antennae (especially funicular segments towards tips) and legs (especially distal portion of tibiae and tarsi). Head with medially erect macrosetae roughly paired, extending through the medial portion of clypeus. Cuticle on the head smooth and shining, with faint shagreenate sculpture, especially towards the posterior margin. Antennae 12 segmented; scapes very long, with scape index above 180, usually above 200 (SI 183–220). Scapes with a dense layer of pubescence. Head is distinctly longer than wide, with cephalic index below 100 (CI 71-94); posterolateral corners rounded, with straight posterior margin. Eyes large relative to head width (REL2 greater than 35); eyes distinctly convex, extending beyond head margin in full frontal view; three small but distinct ocelli present. Mandibles typical for Prenolepis genus-group (Fig. 9), with 5 teeth; mandalus large and anteriorly placed; palps very long (0.70–0.75 mm); palp formula 6:4; segments 3-6 are longest (numbered from basal segment (1) to apical segment (6). Mesosoma distinctly elongated; in profile pronotum and mesonotum long; propodeal dorsal face either nearly flat or moderately convex (Figs 2, 5); propodeum without macrosetae, anteriorly occasionally with a sparse layer of pubescence; pronotal setal count 5–10 (both sides of notum); mesonotal setal count 4-8 (both sides of notum). Petiole cuneate, broadly rounded dorsally, with much longer posterior face and not surpassing the height of the propodeum. Legs distinctly long (profemur length 0.6–1.0 mm). Gaster robust, covered in abundant erect macrosetae.
Paratrechina workers in various views (left to right: full frontal, lateral and dorsal views for figs 1–6): 1–3 Paratrechina longicornis (CASENT0250003) 4–6 Paratrechina zanjensis holotype (SAM-HYM-C020685) 7 Paratrechina longicornis scape (CASENT0250003) 8 Paratrechina zanjensis holotype scape (SAM-HYM-C020685) 9 Paratrechina zanjensis paratype mandible (CASENT0250002).
Paratrechina longicornis (Latreille, 1802) Pantropical tramp, origin uncertain
= Paratrechina currens Motschoulsky, 1863. Junior synonym of Paratrechina longicornis by Emery, 1892: 166. Neotype designated by
LaPolla et al. 2010b : 1.= Paratrechina gracilescens (Nylander, 1856). Synonymy with Paratrechina longicornis by
Roger 1863 : 10.= Paratrechina longicornis hagemanni (Forel, 1901). Junior synonym of Paratrechina longicornis by
Wheeler 1922 : 942. Revived from synonymy by Emery, 1925: 217. Junior synonym of Paratrechina longicornis byLaPolla et al. 2010a : 128.= Paratrechina vagans (Jerdon, 1851). Junior synonym of Paratrechina longicornis by
Dalla Torre 1893 : 179;Forel 1894 : 408.
Paratrechina zanjensis, sp. n. Angola, Mozambique and Tanzania
For a genus-level key see
| 1 | Scapes without erect macrosetae (Fig. 7); in lateral view propodeal dorsal face almost flat (Fig. 2) | Paratrechina longicornis |
| – | Scapes with erect macrosetae (Fig. 8); in lateral view propodeal dorsal face convex (Fig. 5) | Paratrechina zanjensis sp. n. |
http://species-id.net/wiki/Paratrechina_longicornis
Figs 1–3, 7 (worker); Figs 12–17 (male)Scapes without erect macrosetae.
WORKER. Measurements in millimeters (n=4): TL: 2.1-2.5; HW: 0.46-56; HL: 0.49-0.7; EL: 0.17-0.23; SL: 0.98-1.16; PW: 0.34-0.43; WL: 0.82-0.98; PrFL: 0.6-0.9; GL: 0.83-0.9.
Indices: CI: 73-94; REL2: 38-42; SI: 182-226.
Overall coloration pale to very dark brown, often with a distinct blueish iridescent sheen, especially on the mesosoma and gaster. Mandibles, antennae and legs (especially the trochanters of all legs, which are a strongly contrasting very pale yellow-brown) much lighter in color; cuticle smooth and moderately shining with faint shagreenate sculpture, which is most obvious on head and gaster. Head narrow, distinctly longer than broad, with abundant pale (yellow-brown to almost white), erect macrosetae; anterior clypeal margin with a shallow medial indentation; scapes with a dense layer of very fine pubescence but lacking erect macrosetae; eyes large and convex, extending beyond head lateral margin in full frontal view; posterior head margin with rounded posterolateral corners; three distinct ocelli present. Mesosoma with scattered pale erect macrosetae (PSC = 3; MSC = 3-4); in profile pronotum and mesonotum almost flat dorsally, with a broadly angled junction; metanotal area relatively indistinct, medially about 1/5 the length of the mesonotum but longer laterally than medially; dorsum of propodeum almost flat to very shallowly domed, rounding evenly into the short declivitious face; anterolateral portion of dorsal face with some scattered pubescence. Gaster with abundant erect pale macrosetae.
The authors have examined hundreds of specimens of Paratrechina longicornis from around the world for this and other related studies. These are not listed here. The four specimens measured for this study were from: MADAGASCAR: Prov. Mahajanga; Mahavavy River; 6.2 km 145 SE Mitsinjo (CASENT0490165); 16°03.1'S, 45°54.5'E (BLF6931); MAURITIUS: Pte d’Esny; 20°25.52'S, 57°43.44'E (CASENT0055961).
http://zoobank.org/B8FE1331-6242-44AB-8E31-BBE08175E83E
http://species-id.net/wiki/Paratrechina_zanjensis
Figs 4–6, 8, 9 (worker)TANZANIA: Ruvuma Region, Namtumbo District, Mkuju River, 855m, 10.07400S, 36.57959E ± 100m, 27–29.xi.2011, MKU2011-3.1 (P Hawkes, J Fisher) (SAMC: SAM-HYM-C020685). 13 paratypes with same data as holotype, 7 paratypes, TANZANIA: Ruvuma Region, Namtumbo District, Mkuju River, 831m, 10.08380S, 36.57267E ± 100m, 2–4.xii.2011, MKU2011-5.1 (P Hawkes, J Fisher) (AFRC, CASC, MCZC, NMKE, SAMC, USNM).
Scapes with abundant erect macrosetae.
WORKER. Measurements in millimeters (n=18): TL: 2.6-3.2; HW: 0.50-0.59; HL: 0.68-0.77; EL: 0.19-0.23; SL: 1.07-1.24; PW: 0.41-0.5; WL: 0.94-1.20; PrFL: 0.78-0.91; GL: 0.92-1.30.
Indices: CI: 71-79; REL2: 37-44; SI: 198-220.
Overall coloration dark brown with lighter mandibles, antennae (especially funicular segments towards tips) and legs (especially trochanters of mid and hind legs, and distal portions of tibiae and tarsi); cuticle smooth, shining, and with very faint shagreenate sculpture, which is more obvious on head and gaster. Head narrow, distinctly longer than broad, with abundant dark, erect macrosetae; anterior clypeal margin with a shallow medial indentation; scapes with a dense layer of pubescence and scattered erect macrosetae (SSC = 17-29); eyes large and convex, extending beyond head lateral margin in full frontal view; posterior head margin with rounded posterolateral corners; three distinct ocelli present. Mesosoma with scattered dark erect macrosetae (PSC = 2-5; MSC = 2-3); in profile pronotum and mesonotum shallowly convex dorsally, presenting a uniform overall curvature through their junction; metanotal area distinct, about 1/3 the length of the mesonotum; dorsal face of propodeum rounded, with short declivitious face; anterior portion of dorsal face with thin layer of pubescence. Gaster with abundant erect dark macrosetae.
The specific epithet is derived from the ancient Arabic name for the stretch of East African coast that encompasses parts of modern day Kenya, Mozambique, and Tanzania.
ANGOLA: Huambo, Mont. Moko, 1725 m, 12°27.20'S, 15°07.45'E, 7.vi.2007, miombo woodland (BL Fisher) (CASENT0128086); MOZAMBIQUE: Sofala Prov., Gorongosa N.P., Ravine (18.63407S, 34.80689E), 172 m, 12.vi.2012 (P Naskrecki); Sofala Prov., Gorongosa N.P., Gorongosa Mountain, road to waterfall (18.49764S, 34.04975E), 800 m, 19.vi.2012 (GD Alpert).
Very little is known about the natural history of Paratrechina zanjensis; the 23 specimens collected in the Mkuju River region of the Selous Game Reserve in Tanzania were all found in 48-hour pitfall trap samples (present in 8 of a total of 60 samples from two sites separated by about 1.3 km) while none were found in the 40 Winkler-extracted leaf litter samples collected during daytime along the same transects. The two Tanzanian sites in which Paratrechina zanjensis were found were representative of mature dry miombo woodland, while they appeared to be absent from both dry and moist closed canopy forest sites nearby. The Angola and Mozambique specimens were also collected in miombo woodlands; this, in combination with the absence of Paratrechina zanjensis from 15 forest sites surveyed by one of us (PH) in the Eastern Arc Mountains and Coastal Forests of Tanzania, suggests that the species prefers open woodland rather than forest habitats.
The worker of Paratrechina zanjensis can easily be separated from Paratrechina longicornis based on the presence of erect macrosetae on the scapes. There are several other notable differences between the two species (see Figs 1–6). The propodeal dorsal face of Paratrechina zanjensis is more convex than is observed in Paratrechina longicornis. Similarly, the pronotum and to a lesser degree the mesonotum are more convex in Paratrechina zanjensis, being almost flat in Paratrechina longicornis. The metanotal area is also longer and more distinct in Paratrechina zanjensis, being more strongly separated from the mesonotum and propodeum. Paratrechina zanjensis has erect macrosetae that are dark rather than pale as in Paratrechina longicornis, the body is glossier, and largely lacks the opalescent sheen / iridescence that is characteristic of Paratrechina longicornis. Unfortunately, the male and queen for Paratrechina zanjensis remain unknown. Paratrechina zanjensis is superficially similar to Nylanderia silvula (see images in
Paratrechina has been shown to be a distinct lineage within the Prenolepis genus-group (
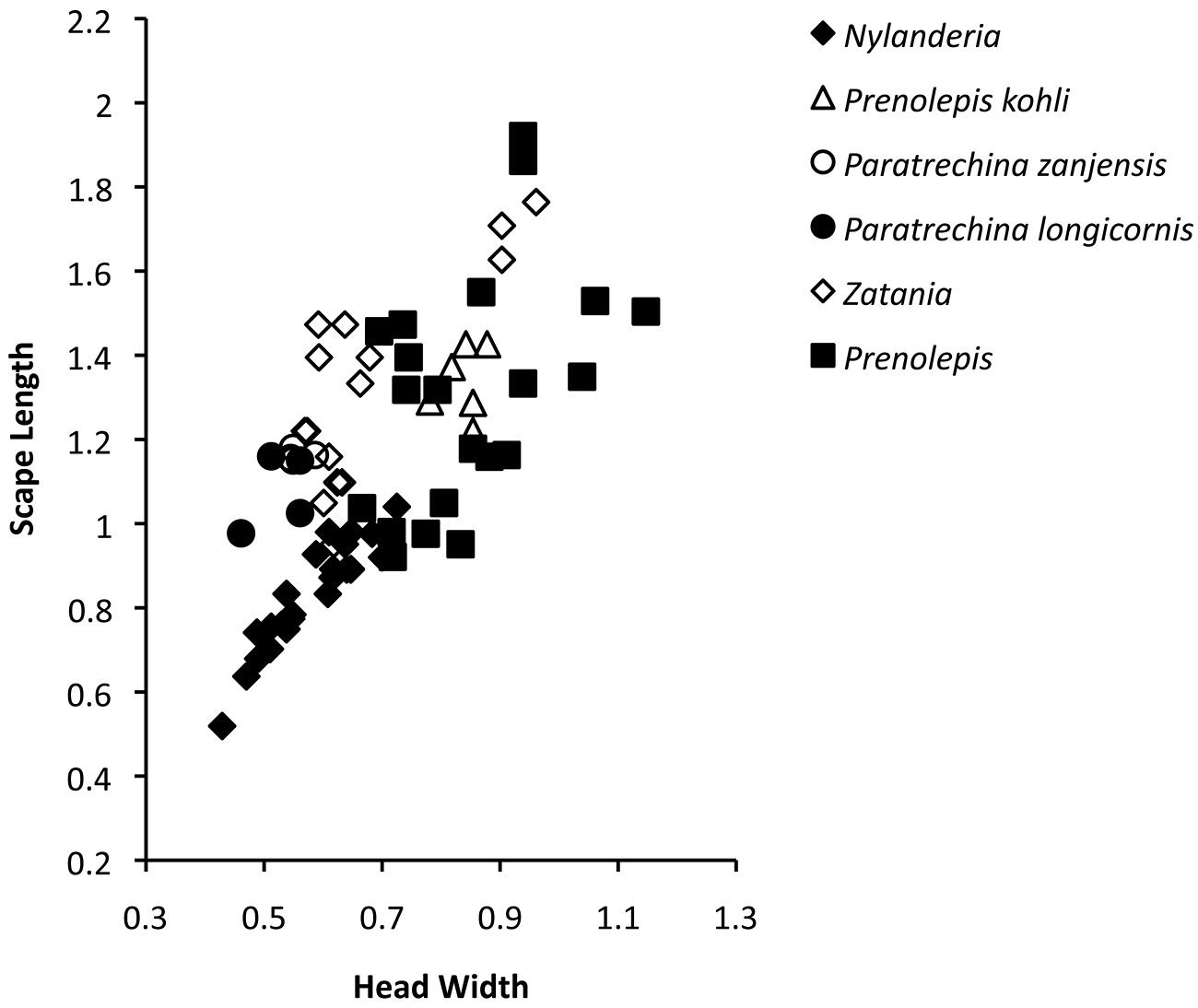
Scape length versus head width of various Prenolepis genus-group taxa. Measurements are based on results from this study and
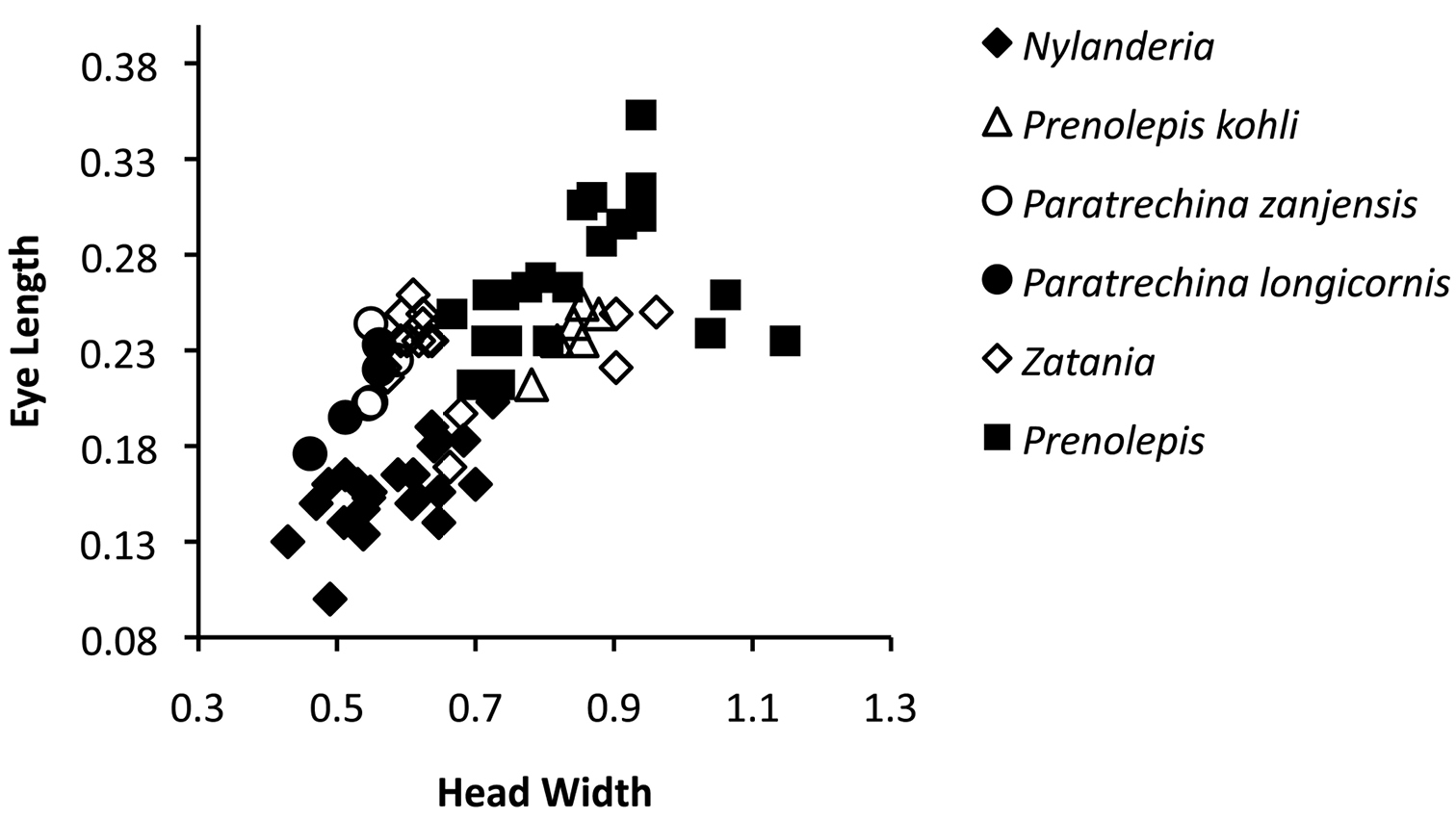
Eye length versus head width of various Prenolepis genus-group taxa. Measurements are based on results from this study and
The similarity of the scapes of Paratrechina, Prenolepis and Zatania extends to other aspects of overall worker morphology within these genera, and these three genera are the most likely members of the Prenolepis genus-group to be confused with each other. This is because workers from these genera generally possess elongated heads, scapes, mesosomata, and legs. Whether or not the elongated state of these morphological features represents the plesiomorphic state (as might be suggested by the basally diverging position of Prenolepis within the clade, see
As was found in Zatania (
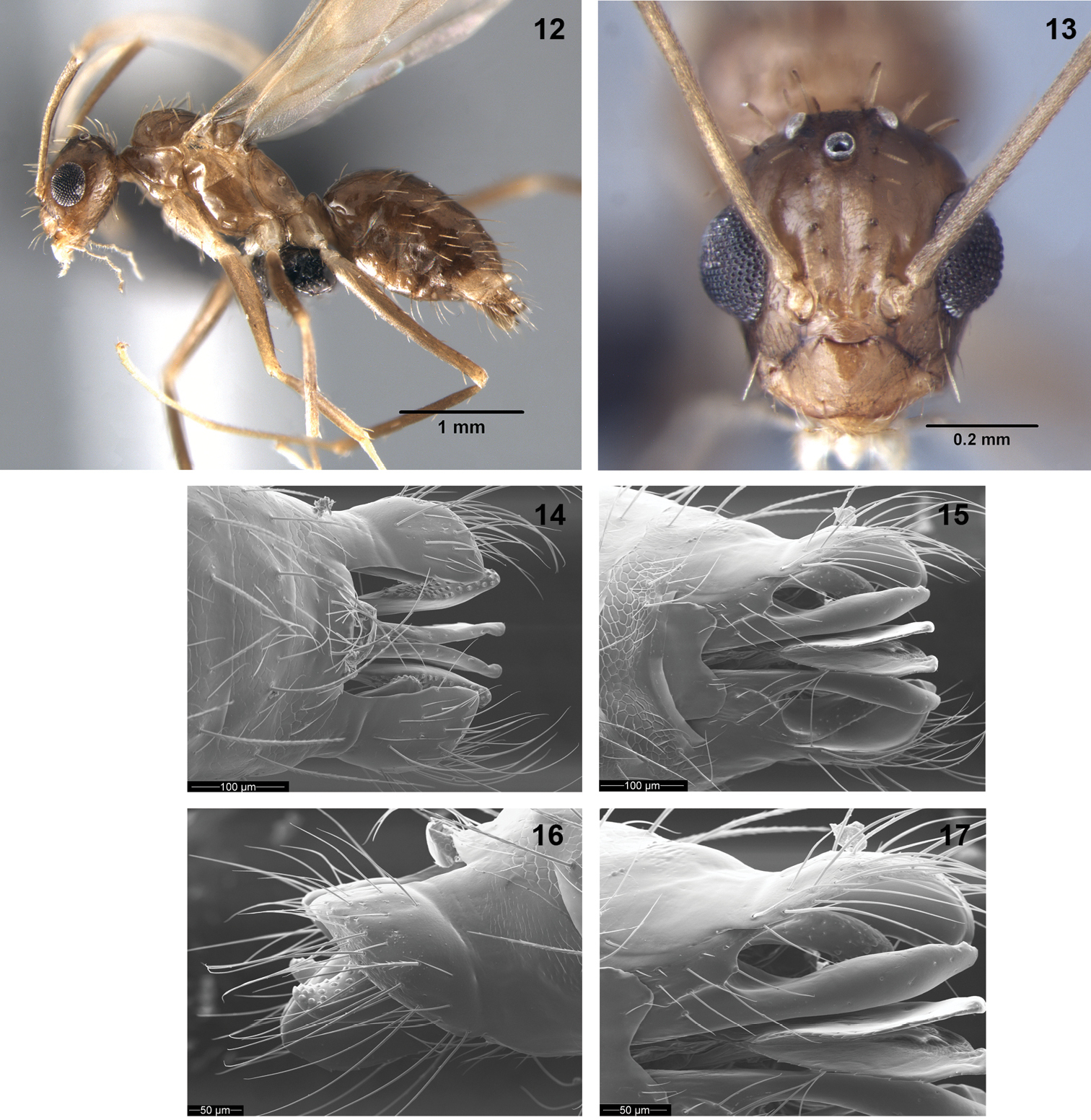
Paratrechina longicornis male (14–17 are scanning electron microscope images): 12 lateral view 13 full frontal view 14 genitalia dorsal view 15 genitalia ventral view 16 genitalia lateral view 17 close-up view of digitus and cuspis.
Paratrechina zanjensis is certainly native to Africa, to date only being found in natural or minimally disturbed habitats. The presence of a new species of Paratrechina in Africa is interesting because Paratrechina longicornis is thought to be native to Asia (although some authors have considered it native to Africa, see
This study was supported in part by the National Science Foundation under grants DEB-0743542 (awarded to JSL). We thank Gary Alpert for images and label data of specimens from Mozambique, and Brian Fisher for images and label data of specimens from Angola.