(C) 2013 Abhineshwar V. Prasad. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Prasad AV, Hodge S (2013) Factors influencing the foraging activity of the allodapine bee Braunsapis puangensis on creeping daisy (Sphagneticola trilobata) in Fiji. Journal of Hymenoptera Research 35: 56–69. doi: 10.3897/JHR.35.6006
Bees, invasive plants, pollinators, South Pacific
There is growing concern regarding the global decline of honey bee populations and the implications of this demise for the pollination of entomophilous crops (
The situations described above give the impression that the presence of some exotic flowering plants may be of benefit by encouraging higher numbers of pollinating species to occur at a site. Outside of agro-ecological systems, many studies have indicated that even flowering plants considered as invasive may have positive effects on insects, especially on nectar and pollen feeding species. For example, in Europe and North America, the exotic highly invasive Himalayan balsam (Impatiens glandulifera Royle) is visited by a high diversity of native pollinating insects, including bumble bees (Bombus spp), solitary bees, and domestic honey bees (Apis mellifera L.) (
Sphagneticola trilobata (L.) Pruski (Asteraceae) is an emerald-green creeping plant that has bright yellow daisy-like flowers. The plant is of Central/South American origin and is now found in many South Pacific island states, where it has become established on disturbed sites, such as waste land, road sides, riverbanks and the sea shore (
The aim of this study was to obtain empirical data on the activity and distribution of Braunsapis puangensis in the Suva area of Fiji and examine its association with Sphagneticola trilobata. We studied spatial patterns on a local scale by recording its presence or absence on patches of Sphagneticola trilobata along roadsides, and carried out long term sampling over 14 months to gain information on patterns in seasonal occurrence. A more detailed study was performed at a single site to investigate daily foraging patterns and examine the effects of environmental conditions on Braunsapis puangensis activity.
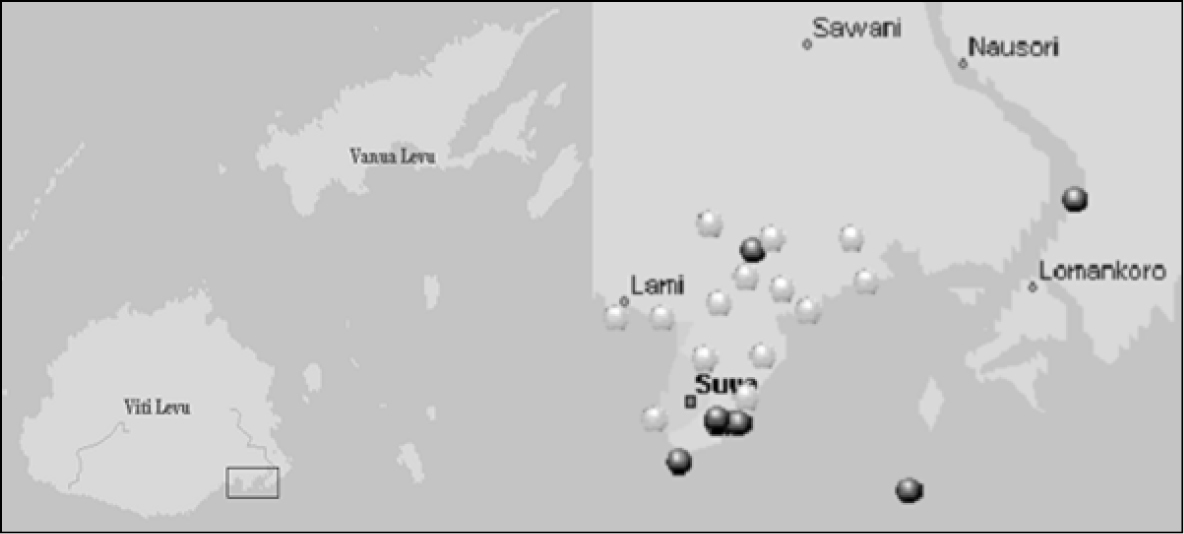
Twenty-two patches of Sphagneticola trilobata were selected to give widespread coverage of the Greater Suva area (including Lami and Nasinu) and the surrounding area (Rewa Delta, Nukulau Island) (Table 1). All the patches of Sphagneticola trilobata sampledwere greater than 4m2 in area and close to roadsides or tracks. Insects were either sampled using a sweep net or obtained directly from the flowers using a battery-powered aspirator. To avoid over sampling the bees, only the presence/absence of Braunsapis puangensis was recordedat some locations. The coordinates of each site were recorded using a geographical positioning system [‘e trex’; Garmin Ltd, Southampton, UK] and these used to plot a map of the sites using Microsoft MapPoint (Figure 1).
The presence or absence of Braunsapis puangensis on twenty two patches of Sphagneticola trilobata in the Greater Suva area visited in April 2011.
| Site no. | Site Identification | Latitude, Longitude | Braunsapis puangensis |
|---|---|---|---|
| 1 | USP, Campus library | -18.150, 178.445 | ✓ |
| 2 | USP, Lower Campus | -18.150, 178.453 | ✓ |
| 3 | USP, Upper Halls | -18.149, 178.445 | ✓ |
| 4 | Colo-i-Suva | -18.091, 178.458 | ✓ |
| 5 | Bowling Club | -18.149, 178.423 | - |
| 6 | Muanikau | -18.150, 178.450 | ✓ |
| 7 | Vatuwaqa Cemetery | -18.141, 178.456 | - |
| 8 | FNU Hospitality | -18.163, 178.431 | ✓ |
| 9 | Lami rubbish dump | -18.114, 178.425 | - |
| 10 | Lami town | -18.114, 178.409 | - |
| 11 | Laucala Beach | -18.112, 178.478 | - |
| 12 | Golf course | -18.127, 178.462 | - |
| 13 | Khalsa Road | -18.087, 178.465 | - |
| 14 | Savura | -18.082, 178.443 | - |
| 15 | Namadi heights | -18.109, 178.446 | - |
| 16 | Nadawa | -18.102, 178.499 | - |
| 17 | Kalabo | -18.087, 178.494 | - |
| 18 | Caubati | -18.104, 178.469 | - |
| 19 | Ram Lakhan Park | -18.128, 178.441 | - |
| 20 | Cunningham Road | -18.100, 178.456 | - |
| 21 | Nukulau Island | -18.173, 178.514 | ✓ |
| 22 | Rewa Delta | -18.074, 178.574 | ✓ |
Maps of major Fiji Islands showing general location of study area, and of Greater Suva indicating locations of patches of Sphagneticola trilobata sampled in the current study. Dark circles indicate presence and white circles indicate absence of Braunsapis puangensis.
Long term monitoring of Braunsapis puangensis was carried out using yellow pan traps placed out under the eaves of a house on The University of the South Pacific (USP) Upper Campus within a patch of Sphagneticola trilobata (and a few other low-lying plants). The traps consisted of 30 cm × 25 cm rectangular plastic bowls (20cm deep) that were half filled with water to which a few drops of household detergent had been added. Sampling was continuous, using two traps at all times which were emptied once each week. The insects collected in each calendar month were then pooled. Collecting was carried out for 14 months, from May 2010 to June 2011.
A single patch of Sphagneticola trilobata on The USP Upper Campus was used to monitor daily activity patterns of Braunsapis puangensis. Activity was estimated by dividing the patch of flowers into three sectors (each 2 m × 2 m) and performing a 30 second count of individuals in each sector. A mean of the three counts was then obtained. Attempts were made not to count the same individual more than once. This process was repeated every hour, from just prior to dawn and to just after dusk, and was repeated over five separate days (during April 2011). Light intensity, relative humidity and temperature were measured on each occasion using electronic meters.
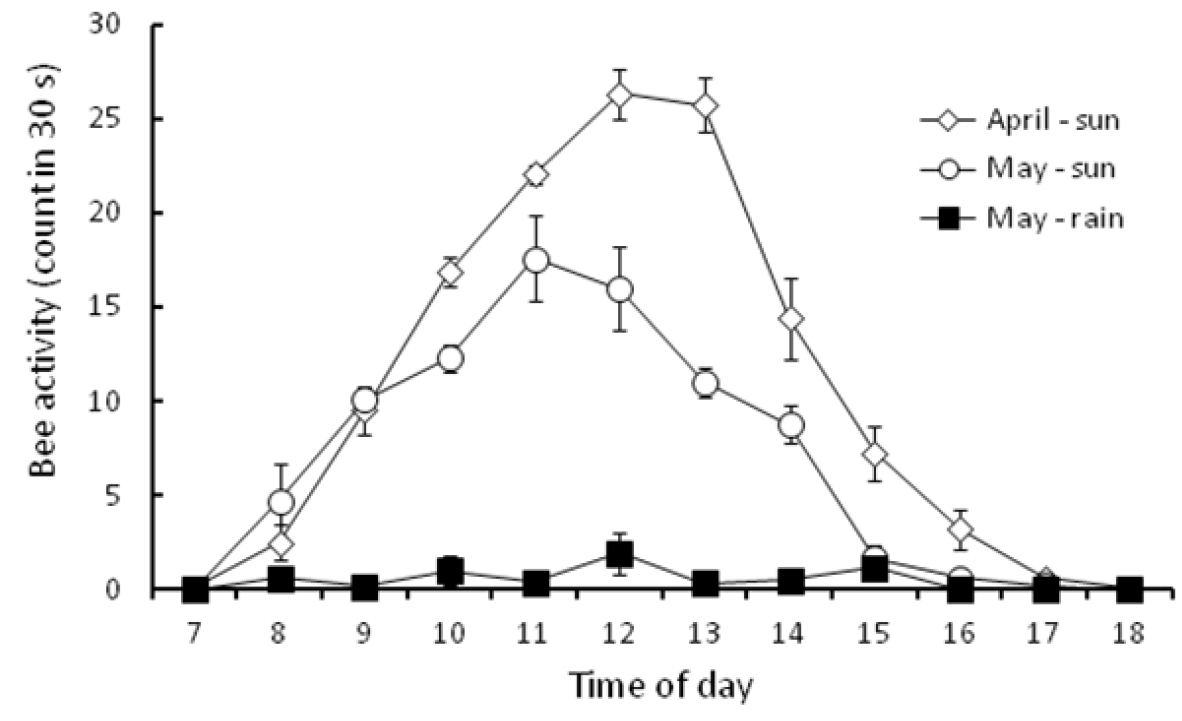
The whole procedure was repeated in May 2011, but on this occasion the activity of the bees was measured during dry periods and during rain until there were five replicates of bee activity for each hourly interval for both dry and wet conditions.
Braunsapis puangensis was recorded at eight of the twenty two sites sampled (Table 1; Figure 1). There was a cluster of sites where Braunsapis puangensis was present at Suva Point, but other sites were quite widespread, from the coastal sites in Laucala and Nukulau Island to the inland forests at Colo-i-Suva (Figure 1).
The activity of Braunsapis puangensis observed in May was generally lower than that observed in the April survey at any given time point, but the daily patterns in activity were similar (Figure 2). In fine weather, the activity of the bees increased steadily from 8am, reached a peak around mid-day and then decreased through the afternoon. No Braunsapis puangensis were observed on the patch of Sphagneticola trilobata on the USP campus prior to 7am and after 6pm.
Braunsapis puangensis activity from 7am to 6pm on a single Sphagneticola trilobata patch at The University of the South Pacific, Laucala Campus (individuals counted in 30 s; mean ± SE, n = 5). Observations were made during sunny weather in April 2011, and during sunny and rain conditions in May 2011.
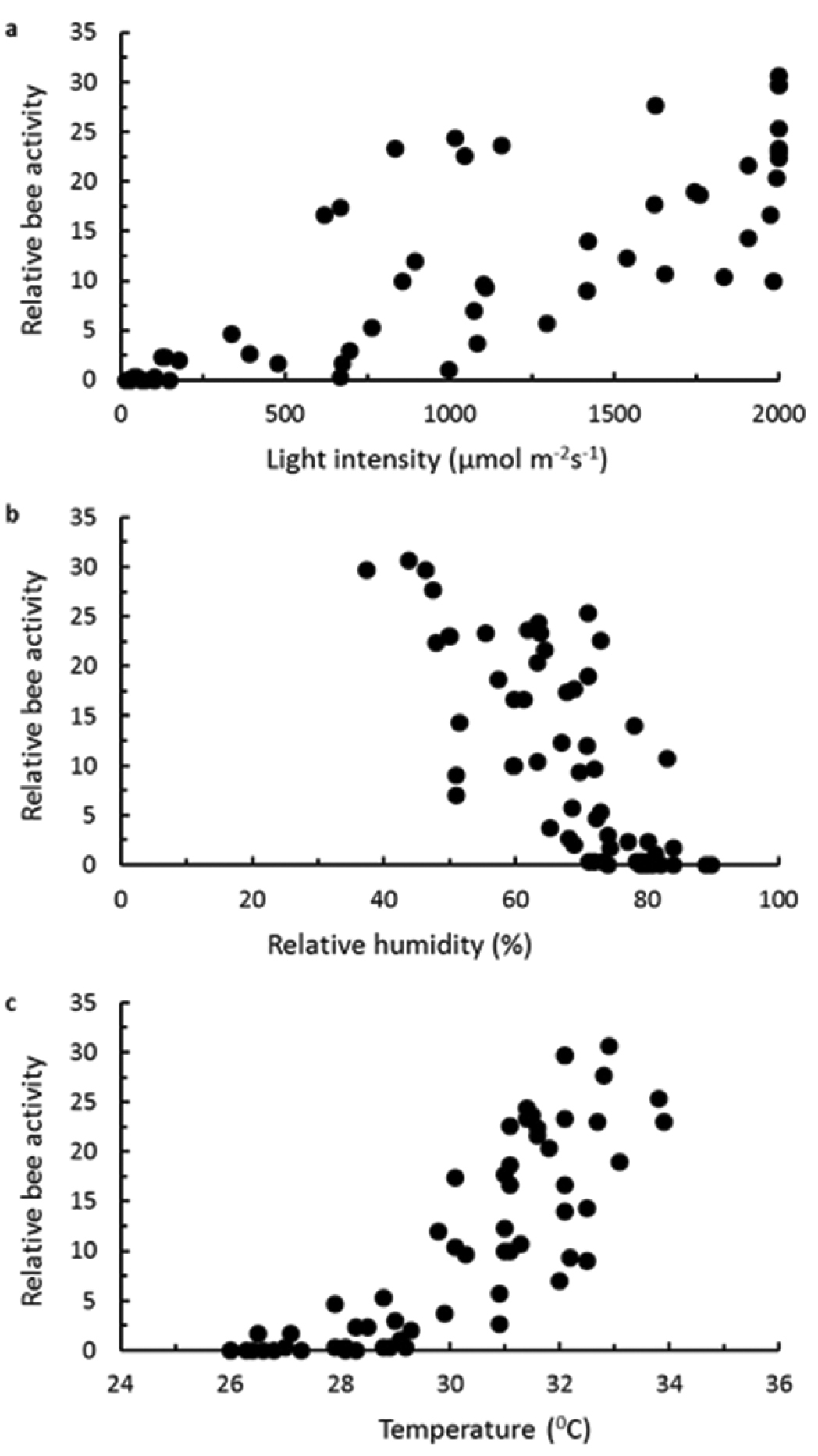
The environmental parameters measured were all co-correlated, with high mid-day temperatures being associated with high light and low relative humidity, and lower temperatures recorded early morning and late afternoon being associated with lower light levels and higher relative humidity (Table 2). Therefore the activity of Braunsapis puangensis displayed an association with a set of environmental conditions, activity having a strong positive correlation with temperature and light intensity, and a strong negative correlation with relative humidity (Figure 3; Table 2). The activity of the bees virtually ceased during periods of rain (Figure 2), although bees were often observed foraging soon after the rain had stopped.
Correlation of environmental factors and activity of Braunsapis puangensis in a single patch of Sphagneticola trilobata on the USPLaucala campus (April 2011). Values given are rs, Spearman’s rank correlation coefficient (n = 60; P < 0.001 in all cases).
| Relative Humidity | Light Intensity | Bee Activity | |
|---|---|---|---|
| Temperature | -0.79 | 0.85 | 0.87 |
| Relative Humidity | - | -0.77 | -0.78 |
| Light Intensity | - | - | 0.86 |
The relationship between activity of Braunsapis puangensis (counts in 30 s) and a light intensity b relative humidity and c temperature at a single patch of Sphagneticola trilobata on the USP Laucala campus during fine weather in April 2011.
Braunsapis puangensis was recorded in all 14 monthly samples, indicating that adults were present and active in the Suva area over the whole annual cycle. However, the use of water traps as a collecting method for these bees was largely unsuccessful. Braunsapis puangensis was only recorded in very low numbers (between 1 and 4 individuals each month: data not shown), even when foraging activity was observed to be high in the surrounding area. Thus, due to the constant low catch, there were no obvious seasonal patterns in Braunsapis puangensis abundance revealed by this method.
The frequency of occurrence of Braunsapis puangensis over the sample sites (8 of 22) was lower than we had anticipated. Our initial expectations were based on observations of foraging activity on patches of Sphagneticola trilobata in or near the USP campus in Laucala Bay, where Braunsapis puangensis was common (see Figure 1), often seen in high numbers (see Figure 2) and found regularly throughout the year. The road-side samples were all taken around mid-day in fine weather so, based on the activity patterns observed for this species, if adults were present they would likely have been active when collecting was performed. Braunsapis puangensis has been observed on Sphagneticola trilobata at other locations on the south of Viti Levu (e.g. Pacific Harbour and Sigatoka; S. Hodge 2011 pers. obs.), and
The daily activity patterns of Braunsapis puangensis are similar to those recorded for other bee species.
Solitary and/or native bee species are considered an important resource in terms of pollination of crop species (
There has been some fairly wide-ranging, albeit sporadic, published work on the Hymenoptera of Fiji.
Many thanks to Dinesh Kumar for technical assistance and Scott Groom for help with specimen identification. Funding for AVP was provided by The Faculty of Science, Technology and Environment of The University of the South Pacific.