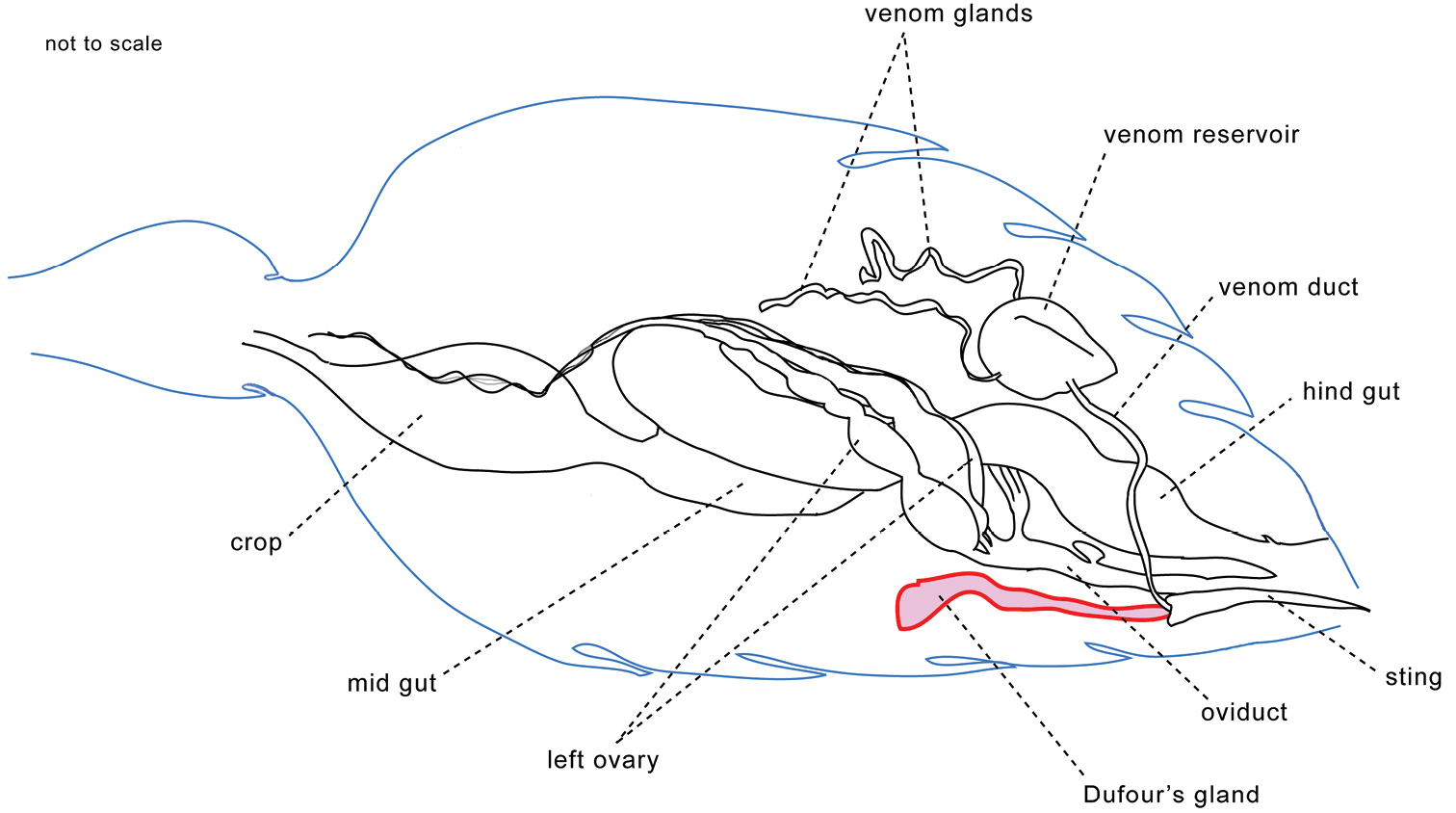
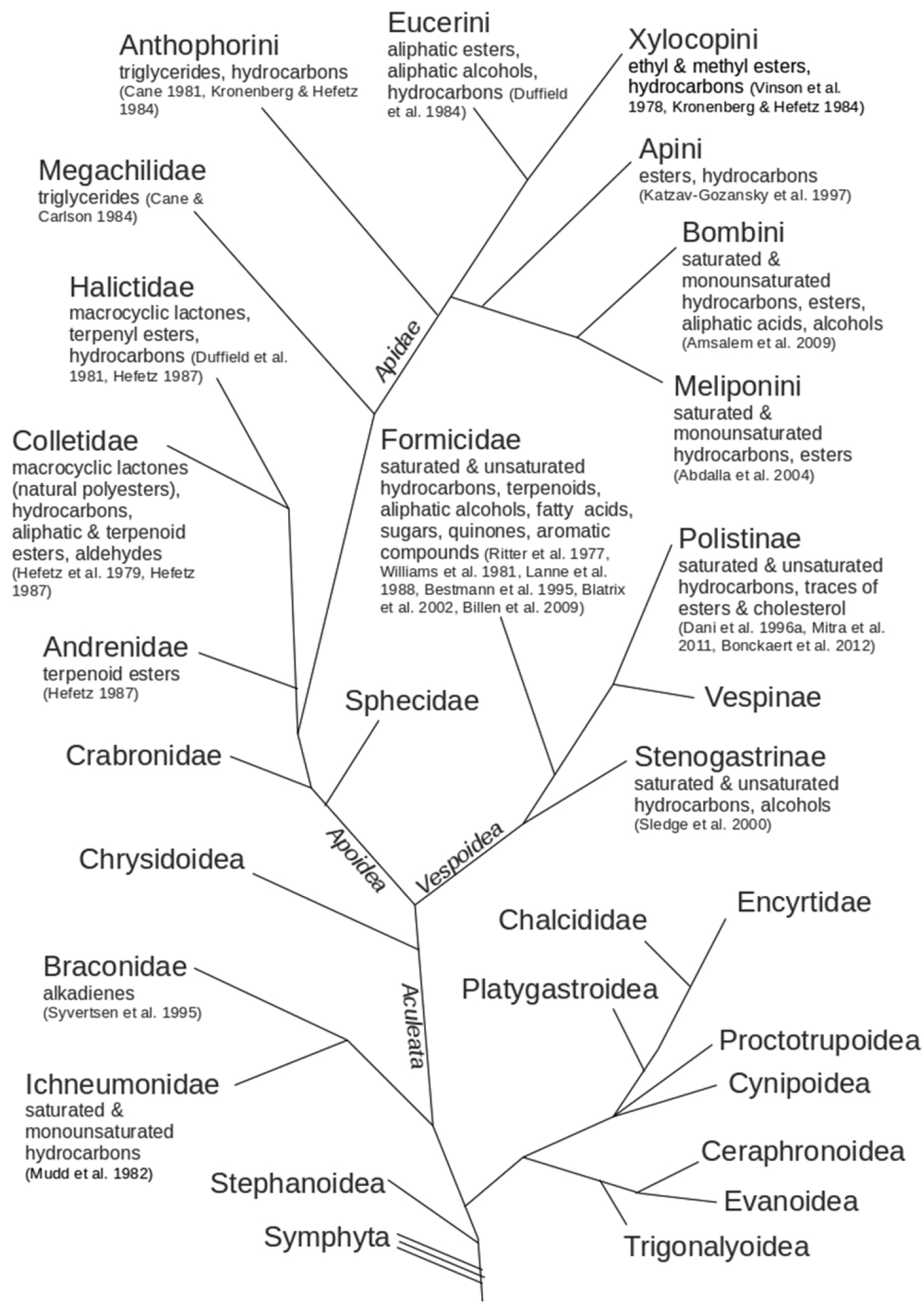
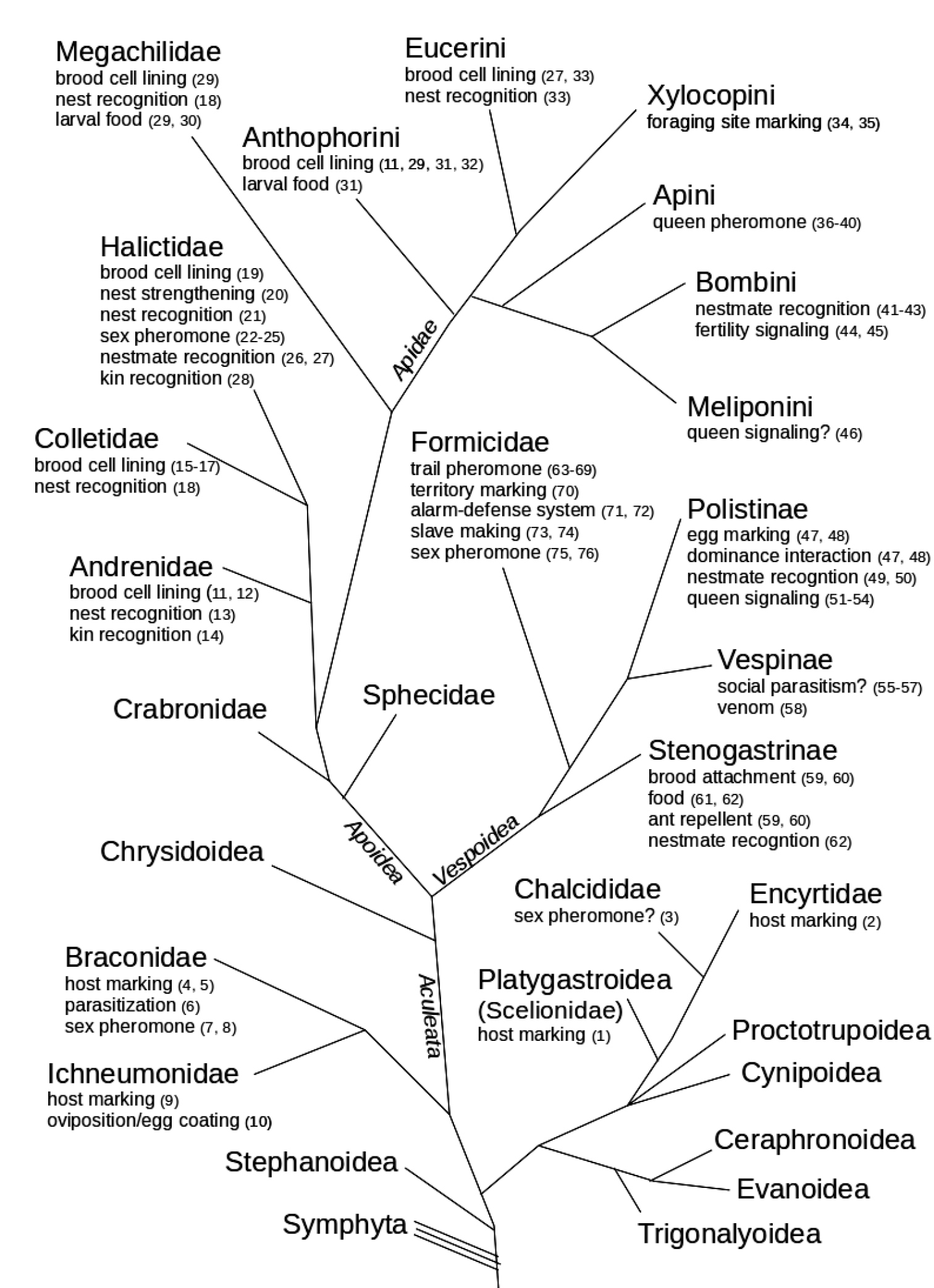
ReferencesAbdalla FC, Cruz-Landim C da (2001) Dufour’s glands in Hymenopterans (Apidae, Formicidae, Vespidae): a review. Revista Brasileira de Biologia 61: 95-106. doi:
10.1590/S0034-71082001000100013Abdalla FC, Cruz-Landim C da (2004) Occurrence, morphology and ultrastructure of the Dufour gland in
Melipona bicolor Lepeletier (Hymenoptera, Meliponini). Revista Brasileira de Entomologia 48: 9-19. doi:
10.1590/S0085-56262004000100002Abdalla FC, Jones GR, Morgan D, Cruz-Landim C da (2004) Chemical composition of the Dufour gland secretion in queens of
Melipona bicolor (Hymenoptera, Meliponini). Journal of the Brazilian Chemical Society 15: 621-625. doi:
10.1590/S0103-50532004000500002Abdalla FC, Velthius H, Cruz-Landim C da, Duchateau MJ (1999) Changes in the morphology and ultrastructure of the Dufour’s gland during the life cycle of the bumble bee queen,
Bombus terrestris (Hymenoptera: Bombini). Netherlands Journal of Zoology 49: 251–261. doi:
10.1163/156854299X00173Albans KR, Aplin RT, Brehcist J, Moore JF, O’Toole C (1980) Dufour’s gland and its role in secretion of nest cell lining in bees of the genus
Colletes (Hymenoptera: Colletidae). Journal of Chemical Ecology 6: 549-564. doi:
10.1007/BF00987667Amsalem E, Hefetz A (2010) The appeasement effect of sterility signaling in dominance contests among
Bombus terrestris workers. Behavioral Ecology and Sociobiology 64: 1685-1694. doi:
10.1007/s00265-010-0982-4Amsalem E, Twele R, Francke W, Hefetz A (2009) Reproductive competetion in the bumble-bee
Bombus terrestris: do workers advertise sterility? Proceedings of the Royal Society of London B 276: 1295–1304. doi:
10.1098/rspb.2008.1688Ayasse M, Birnbaum J, Tengö J, van Doorn A, Taghizadeh T, Francke W (1999) Caste and colony specific chemical signals on eggs of the bumble bee,
Bombus terrestris L. (Hymenoptera: Apidae). Chemoecology 9: 119-126. doi:
10.1007/s000490050042Ayasse M, Engels W, Hefetz A, Lübke G, Francke W (1990a) Ontogenetic patterns in amounts and proportions of Dufour’s gland volatile secretions in virgin and nesting queens of
Lasioglossum malachurum (Hymenoptera: Halictidae). Zeitschrift für Naturforschung, C. 45: 709–714.
Ayasse M, Engels W, Hefetz A, Tengö J, Lübke G, Francke W (1993) Ontogenetic patterns of volatiles identified in Dufour’s gland extracts from queens and workers of the primitively eusocial halictine bee,
Lasioglossum malachmum (Hymenoptera: Halictidae). Insectes Sociaux 40: 41-58. doi:
10.1007/BF01338831Ayasse M, Leys R, Pamilo P, Tengö J (1990b) Kinship in communally nesting
Andrena (Hymenoptera; Andrenidae) bees is indicated by composition of Dufour’s gland secretions. Biochemical Systematics and Ecology 18: 453-460. doi:
10.1016/0305-1978(90)90092-TAyasse M, Paxton RJ, Tengö J (2001) Mating behavior and chemical communication in the order Hymenoptera. Annual Review of Entomology 46: 31-78. doi:
10.1146/annurev.ento.46.1.31Baker J, Howard R, Morrill W, Meers S, WeaverD (2005) Acetate esters of saturated and unsaturated alcohols (C12–C20) are major components in Dufour glands of
Bracon cephi and
Bracon lissogaster (Hymenoptera: Braconidae), parasitoids of the wheat stem sawfly,
Cephus cinctus (Hymenoptera: Cephidae). Biochemical Systematics and Ecology 33: 757-769. doi:
10.1016/j.bse.2004.12.025Barr-Nea L, Rosenberg P, Ishay J (1976) The venom apparatus of
Vespa orientalis: morphology and cytology. Toxicon 14: 65-68. doi:
10.1016/0041-0101(76)90121-5Barrows EM (1975b) Mating behaviour in halictine bees. III. Copulatory behaviour and olfactory communication. Insectes Sociaux 22: 307-331. doi:
10.1007/BF02223079Bestmann HJ, Janssen E, Kern F, Liepold B, Hölldobler B, Boveri T (1995) All-trans geranyl geranyl acetate and geranylgeraniol, recruitment pheromone components in the Dufour gland of the ponerine ant
Ectatomma ruidum Pheromones, 100 [1]. Naturwissenschaften 82: 334-336. doi:
10.1007/BF01131530Billen J (1986a) Morphology and ultrastructure of the Dufour’s and venom gland in the ant,
Myrmica rubra (L.) (Hymenoptera: Formicidae). International Journal of Insect Morphology and Embryology 15: 13-25. doi:
10.1016/0020-7322(86)90003-6Billen J, Morgan DE, Drijfhout F, Farnier K (2009) Unusual structural and chemical characteristics of the Dufour gland in the ant
Meranoplus diversus. Physiological Entomology 34: 93-97. doi:
10.1111/j.1365-3032.2008.00659.xBillen JPJ (1982) The Dufour gland closing apparatus in
Formica sanguinea Latreille (Hymenoptera, Formicidae). Zoomorphology 99: 235-244. doi:
10.1007/BF00312297Billen JPJ (1986b) Comparative morphology and ultrastructure of the Dufour gland in ants (Hymenoptera: Formicidae). Entomologia Generalis 11: 165-181.
Billen JPJ (1987) New structural aspects of the Dufour’s and venom glands in social insects. Naturwissenschaften 74: 340-341. doi:
10.1007/BF00367931Blatrix R, Schulz C, Jaisson P, Francke W, Hefetz A (2002) Trail pheromone of ponerine ant
Gnamptogenys striatula: 4-methylgeranyl esters from Dufour’s gland. Journal of Chemical Ecology 28: 2557-2567. doi:
10.1023/A:1021444321238Bonckaert W, Drijfout FP, D’Ettorre P, Billen J, Wenseelers T (2012) Hydrocarbon signatures of egg maternity, caste membership and reproductive status in the common wasp. Journal of Chemical Ecology 38: 42-51. doi:
10.1007/s10886-011-0055-9Brady SG, Larkin L, Danforth BN (2009) Bees, ants, and stinging wasps (Aculeata). In: Hedges SB, Kumar S (Eds) The timetree of life. Oxford University Press, 264-269.
Brooks RW, Cane JH (1984) Origin and chemistry of the secreted nest entrance lining of
Halictus hesperus (Hymenoptera: Apoidae). Journal of Kansas Entomological Society 7: 161-165.
Brothers DJ (1998) Phylogeny and evolution of wasps, ants and bees (Hymenoptera, Chrysidoidea, Vespoidea and Apoidea). Zoologica Scripta 28: 233-250. doi:
10.1046/j.1463-6409.1999.00003.xBrunet PCJ (1952) The formation of the ootheca by
Periplaneta americana II the structure and function of the left colleterial gland. Quarterly Journal of Microscopical Science 93: 47–69.
Cammaerts MC, Morgan ED, Tyler R (1977) Territorial marking in the ant
Myrmica rubra L. (Formicidae). Biology of Behaviour 2: 263-272.
Cane JH (1981) Dufour’s gland secretion in the cell lining of bees (Hymenoptera: Apoidea). Journal of Chemical Ecology 7: 403-410. doi:
10.1007/BF00995762Cane JH (1983) Preliminary chemosystematics of the Andrenidae and exocrine lipid evolution of the short-tongued bees (Hymenoptera: Apoidea). Systematic Zoology 32: 417-430. doi:
10.2307/2413168Cane JH, Carlson RG (1984) Dufour’s gland triglycerides from
Anthophora,
Emphoropsis (Anthophoridae) and
Megachile (Megachilidae) bees (Hymenoptera: Apoidea). Comparative Biochemistry and Physiology B 78: 769-772. doi:
10.1016/0305-0491(84)90132-9Cardinal S, Straka J, Danforth B (2010) Comprehensive phylogeny of apid bees reveals theevolutionary origins and antiquity of cleptoparasitism. Proceedings of the National Academy ofSciences USA 107: 16207-16211. doi:
10.1073/pnas.1006299107Carlet G (1884) Sur le venin des Hyménopteres et ses organes sécréteurs. C R Academy of Science, Paris 98, 1550–1551.
Cervo R, Dani FR, Zanetti P, Massolo A, Turillazzi S (2002) Chemical nestmate recognition in a stenogastrine wasp,
Liostenogaster flavolineata (Hymenoptera Vespidae). Ethology Ecology and Evolution 14: 351-363. doi:
10.1080/08927014.2002.9522736Chaudhuri A, Sinha AK (1994) Colleterectomy and its impact on some reproductive behaviour of the tropical Tasar silk moth,
Antheraea mylitta Drury (Lepidoptera: Saturniidae). Invertebrate Reproduction and Development 26: 145-152. doi:
10.1080/07924259.1994.9672411Dani FR, Morgan ED, Turillazzi S (1996a) Dufour gland secretion of
Polistes wasp: chemical composition and possible involvement in nestmate recognition (Hymenoptera: Vespidae). Journal of Insect Physiology 42: 541-548. doi:
10.1016/0022-1910(95)00136-0Dani FR, Fratini S, Turillazzi S (1996b) Behavioural evidence for the involvement of Dufour’s gland secretion in nestmate recognition in the social wasp
Polistes dominulus (Hymenoptera: Vespidae). Behavioral Ecology and Sociobiology 38: 311-319. doi:
10.1007/s002650050247Davis RB, Baldauf SL, Mayhew PJ (2010) The origins of species richness in the Hymenoptera: insights from a family-level supertree. BMC Evolutionary Biology 10: 109. doi:
10.1186/1471-2148-10-109de Freitas MDRT, MendonÇa ADL, Nascimento RRD, Sant’Ana AEG (2004) Behavioural evidence for a female sex pheromone in
Cotesia flavipes (Hymenoptera: Braconidae). Physiological Entomology 29: 183–187. doi:
10.1111/j.0307-6962.2004.0385.xDowning HA (1991) A role of the Dufour’s gland in the dominance interactions of the paper wasp,
Polistes fuscatus (Hymenoptera: Vespidae). Journal of lnsect Behavior 4: 557-565. doi:
10.1007/BF01048070Downing HA, Jeanne RL (1983) Correlation of season and dominance status with activity of exocrine glands in
Polistes fuscatus (Hymenoptera: Vespidae). Journal of the Kansas Entomological Society 56: 387-397.
D’Rozario AM (1942) On the development and homologies of the genitalia and their ducts in Hymenoptera. Transactions of the Royal Entomological Society, London 92: 363-415. doi:
10.1111/j.1365-2311.1942.tb01211.xDuffield KM, Fernandes A, Lamb C, Wheeler JW, Eickwort GC (1981) Macrocyclic lactones and isopentenyl esters in the Dufour’s gland secretion of halictine bees (Hymenoptera: Halictidae). Journal of Chemical Ecology 7: 319-331. doi:
10.1007/BF00995755Duffield RM, Harrison SE, Maglott D, Ayorinde FO, Wheeler JW (1983) Exocrine secretion of bees. V. Terpenoid esters in the Dufour’s gland secretions of
Panurginus bees (Hymenoptera: Adrenidae). J Chem Ecol 9: 277-283. doi:
10.1007/BF00988045Duffield RM, Wheeler JW, Eickwort GC (1984) Sociochemicals of bees. In: Bell WJ, Cared RT (Eds) Chemical Ecology of lnsects. Chapman and Hall, London: 387-328.
Dufour L (1841) Recherches anatomiques et physiologiques sur les Orthoptères, les Hyménoptères et les Neuroptères. Mémoires de l’Académie des Sciences, Institute de France. Vol 7: 265-647.
Eller FJ, Bartelt RJ, Jones RL, Kulman HM (1984) Ethyl (
Z)-9-hexadecenoate a sex pheromone of
Syndipnus rubiginosus, a sawfly parasitoid. Journal of Chemical Ecology 10: 291-300. doi:
10.1007/BF00987857Fisher RM, Greenwood DR, Shaw GJ (1993) Host recognition and the study of a chemical basis for attraction by cuckoo bumble bees (Hymenoptera: Apidae). Journal of Chemical Ecology 19: 771-786. doi:
10.1007/BF00985008Fortunato A, Turillazzi S (2012) Dufour’s gland possible role in the evolution of sting morphology and function in hover wasps (Hymenoptera Stenogastrinae). Arthropod Structure and Development 41: 259-264. doi:
10.1016/j.asd.2012.02.007Frankie GW, Vinson SB (1977) Scent marking of passion flowers in Texas by female
Xylocopa virginica texana (Hymenoptera: Anthophoridae). Journal of Kansas Entomological Society 50: 613-625.
Gnatzy W, Volknandt W, Schulz S (2004) Dufour gland of the digger wasp
Liris niger: structure and developmental and biochemical aspects. Cell and Tissue Research 315: 125-138. doi:
10.1007/s00441-003-0813-2Greene MJ, Gordon DM (2007) How patrollers set foraging direction in harvester ants. The American Naturalist 170: 943-948. doi:
10.1086/522843Grimaldi D, Engel MS (2005) Evolution of the insects. Cambridge University Press.
Guédot C, Pitts-Singer TL, Buckner JS, Bosch J, Kemp WP (2006) Olfactory cues and nest recognition in the solitary bee
Osmia lignaria. Physiological Entomology Volume 31: 110-119. doi:
10.1111/j.1365-3032.2005.00490.xGuillot FS, Joiner RL, Vinson SB (1974) Host discrimination: isolation of hydrocarbons from the Dufour’s gland of a braconid parasitoid. Annals of the Entomological Society of America 67: 720-21.
Haak U, Hölldobler B, Bestmann HJ, Kern F (1996) Species-specificity in trail pheromones and Dufour’s gland contents of
Camponotus atriceps and
C. floridanus (Hymenoptera: Formicidae). Chemoecology 7: 85-93. doi:
10.1007/BF01239485Hefetz A, Bergstrom G, Tengö J (1986) Species, individual and kin specific blends in Dufour’s gland secretion of halictine bees: chemical evidence. Journal of Chemical Ecology 12: 197-208. doi:
10.1007/BF01045603Hefetz A, Eickwort GC, Blum MS, Cane J, Bohart GE (1982) A comparative study of the exocrine products of cleptoparasitic bees (
Holcopasites) and their hosts (
Calliopsis) (Hymenoptera: Anthophoridae, Andrenidae). Journal of Chemical Ecology 8: 1389-1397. doi:
10.1007/BF01403102Hefetz A, Fales HM, Batra SWT (1979) Natural polyesters: Dufour’s gland macrocyclic lactones in the brood cell laminesters in
Colletes bees. Science 204: 415-417. doi:
10.1126/science.204.4391.415Hefetz A, Bergström G, Tengö J (1986) Journal of Chemical Ecology 12: 197-208. doi:
10.1007/BF01045603Heraty J, Ronquist F, Carpenter JM, Hawks D, Schulmeister S, Dowling AP, Murray D, Munro J, Wheeler WC, Schiff N, Sharkey M (2011) Evolution of the hymenopteran megaradiation. Molecular Phylogenetics and Evolution 60: 73-88. doi:
10.1016/j.ympev.2011.04.003Hermann HR (1969) The hymenoptera poison apparatus: evolutionary trends in three closely related subfamilies of ants (Hymenoptera: Formicidae). Georgia Entomological Society 4: 123–141.
http://hdl.handle.net/10199/1584Hermann HR, Blum MS (1967a) The morphology and histology of the hymenopterous poison apparatus, II:
Pogonomyrmex badius (Formicidae). Annals of the Entomological Society of America 60: 661-668.
Hermann HR, Blum MS (1967b) The morphology and histology of the hymenopterous poison apparatus, III:
Eciton hamatum (Formicidae). Annals of the Entomological Society of America 60: 1282-1291.
Hermann HR, Blum MS (1981) Defensive mechanisms in social Hymenoptera. In: Hermann HR (Ed) Social Insects, 2
nd vol. Academic Press Inc., New York, 77-197.
Hoell HV, Doyen JT, Purcell AH (1998) Introduction to insect biology and diversity. Oxford University Press, 320.
Hölldobler B, Wilson EO (1970) Recruitment trails in the harvester ant
Pogonomyrmex badius. Psyche 77: 385-399.doi:
10.1155/1970/38470Hölldobler B, Wilson EO (1990) The ants. Springer-Verlag, Berlin.
Hölldobler B, Wust M (1973) Ein sexualpheromon bei der Pharaoameise
Monomorium pharaonis (L.). Zeitschrift für Tierpsychologie 32: 1-9. doi:
10.1111/j.1439-0310.1973.tb01092.xHoward RW, Baker JE (2003) Morphology and chemistry of Dufour glands in four ectoparasitoids:
Cephalonomia tarsalis,
C. waterston i (Hymenoptera: Bethylidae),
Anisopteromalus calandrae, and
Pteromalus cerealellae (Hymeoptera: Pteromalidae). Comparative Biochemistry and Physiology B 135: 153-167. doi:
10.1016/S1096-4959(03)00076-9Howard RW, Baker JE, Morgan ED (2003) Novel diterpenoids and hydrocarbons in the Dufour gland of the ectoparasitoid
Habrobracon hebetor (Say) (Hymenoptera: Braconidae). Archives of Insect Biochemistry and Physiology 54: 95-109. doi:
10.1002/arch.10104Huang F, Shi M, Chen Y-F, Cao T-T, Chen X-X (2008) Oogenesis of
Diadegma semiclausum (Hymenoptera: Ichneumonidae) and its associated polydnavirus. Microscopy Research and Technique 71: 676-683. doi:
10.1002/jemt.20594Hubbard SF, Marris G, Reynolds A, Rowe GW (1987) Adaptive patterns in the avoidance of superparasitism by solitary parasitic wasps. Journal of Animal Ecology 56: 387-401. doi:
10.2307/5055Jeanne RL (1977) Behavior of the obligate social parasite,
Vespula arctica (Hymenoptera: Vespidae). Journal of the Kansas Entomological Society 50: 541-577.
Jeanson R, Ratnieks FLW, Deneubourg J-L (2003) Pheromone trail decay rates on different substrates in the Pharaoh’s ant,
Monomorium pharaonis. Physiological Entomology 28: 192-198. doi:
10.1046/j.1365-3032.2003.00332.xJin YX, Chen YY, Jiang YH, Xu MK (2006) Proteome analysis of the silkworm (
Bombyx mori L.) colleterial gland during different developmental stages. Archives of Insect Biochemistry and Physiology 61: 42-50. doi:
10.1002/arch.20095Katzav-Gozansky T, Hefetz A, Soroker V (2007) Brain modulation of Dufour’s gland ester biosynthesis in vitro in the honeybee (
Apis mellifera). Naturwissenschaften 94: 407-411. doi:
10.1007/s00114-006-0206-yKatzav-Gozansky T, Soroker V, Hefetz A (1997) The biosynthesis of Dufour’s gland constituents in queens of the honeybee (
Apis mellifera). Invertebrate Neuroscience 3: 239-243. doi:
10.1007/BF02480380Katzav-Gozansky T, Soroker V, Hefetz A (2002) Honeybees Dufour’s gland – idiosyncrasy of a new queen signal. Apidologie 33: 525-537. doi:
10.1051/apido:2002035Kronenberg S, Hefetz A (1984) Comparative analysis of Dufour’s gland secretions of two carpenter bees (Xylocopinae: Anthophoridae) with different nesting habits. Comparative Biochemistry and Physiology B 79: 321-425. doi:
10.1016/0305-0491(84)90399-7Landolt PJ, Akre RD (1979) Occurrence and location of exocrine glands in some social Vespidae (Hymenoptera). Annals of the Entomological Society of America 72: 141-148.
Lanne BS, Bergström G, Löfqvist J (1988) Dufour gland alkenes from the four ant species:
F. polyctena,
F. lugubris,
F. truncorum, and
F. uralensis. Comparative Biochemistry and Physiology 91B: 729–734. doi:
10.1016/0305-0491(88)90200-3Lawrence PO, Akin D (1990) Virus-like particles from the poison glands of the parasitic wasp
Biosteres longicaudatus (Hymenoptera: Braconidae). Canadian Journal of Zoology 68: 539–546. doi:
10.1139/z90-079Lello de E (1971a) Adxenal glands of the sting apparatus of bees: Anatomy and histology. I. (Hymenoptera: Colletidae and Andrenidae). Journal of Kansas Entomological Society 44: 5-13.
Lello de E (1971b) Adxenal glands of the sting apparatus of bees: Anatomy and histology. II. (Hymenoptera: Halictidae). Journal of Kansas Entomological Society 44: 14-20.
Malka O, Shnieor S, Katzav-Gozansky T, Hefetz A (2008) Aggressive reproductive competition among hopelessly queenless honeybee workers triggered by pheromone signaling. Naturwissenschaften 95: 553-559. doi:
10.1007/s00114-008-0358-zMarris GC, Hubbard SF, Scrimgeour C (1996) The perception of genetic similarity by the solitary parthenogenetic parasitoid
Venturia canescens, and its effects on the occurrence of superparasitism. Entomologia Experimentalis et Applicata 78: 167-174. doi:
10.1007/BF00187513Martin SJ, Châline N, Oldroyd BP, Jones GR, Ratnieks FLW (2004) Egg marking pheromones of anarchistic worker honeybees (
Apis mellifera). Behavioral Ecology 15: 839-844. doi:
10.1093/beheco/arh089Maschwitz U, Kloft W (1971) Morphology and function of the venom apparatus of insects-bees, wasps, ants and caterpilars. In: Bucherl W, Buckley EE (Eds) Venomous AnimaIs and their venoms, 3
rd vol. Academic Press, New York, 1-59.
Mehrnejad MR, Copland MJW (2007) Host discrimination by the endoparasitoid
Psyllaephagus pistaciae (Hymenoptera: Encyrtidae): a case of time-dependent ability. Biocontrol Science and Technology 17: 401-411. doi:
10.1080/09583150701309196Mitra A, Gadagkar R (2011) Can Dufour’s gland compounds honestly signal fertility in the primitively eusocial wasp
Ropalidia marginata? Naturwissenschaften 98: 157–161. doi:
10.1007/s00114-010-0749-9Mitra A, Gadagkar R (2012a) Queen signal should be honest to be involved in maintenance of eusociality: chemical correlates of fertility in
Ropalidia marginata. Insectes Sociaux 59: 251–255. doi:
10.1007/s00040-011-0214-6Mitra A, Gadagkar R (2012b) Road to royalty – transition of potential queen to queen in the primitively eusocial wasp
Ropalidia marginata. Ethology 118: 694-702. doi:
10.1111/j.1439-0310.2012.02059.xMitra A, Saha P, Chaoulideer ME, Bhadra A, Gadagkar R (2011) Chemical communication in
Ropalidia marginata: Dufour’s gland contains queen signal that is perceived across colonies and does not contain colony signal. Journal of Insect Physiology 57: 280-284. doi:
10.1016/j.jinsphys.2010.11.014Mitra A, Gadagkar R (in press) Dufour’s gland and cuticle in the social wasp
Ropalidia marginata contain the same hydrocarbons in similar proportions. Journal of Insect Science.
Mori A, Grasso DA, Visicchio R, Le Moli F (2000) Colony founding in
Polyergus rufescens: the role of the Dufour’s gland. Insectes Sociaux 47: 7-10. doi:
10.1007/s000400050002Mudd A, Fisher RC, Smith MC (1982) Volatile hydrocarbons in the Dufour’s gland of the parasite
Nemestris canescens (Grav.) (Hymenoptera: Ichneumonidae). Journal of Chemical Ecology 8: 1035-1042. doi:
10.1007/BF00987884Norden BB, Batra SWT, Fales HM, Hefetz A, Shaw JC (1980)
Anthophora bees; unusual glycerides from maternal Dufour’s glands serve as larval food and cell lining. Science 207: 1095–1097. doi:
10.1126/science.207.4435.1095Oldham N, Billen J, Morgan ED (1994) On the similarity of the Dufour gland secretion and the cuticular hydrocarbons of some bumblebees. Physiological Entomology 19: 115-123. doi:
10.1111/j.1365-3032.1994.tb01084.xOldroyd BP, Ratnieks FLW, Wossler TC (2002) Egg-marking pheromones in honey-bees
Apis mellifera. Behavioral Ecology Sociobiology 51: 590-591. doi:
10.1007/s00265-002-0480-4Patricio E, Cruz Lopez L, Maile R, Morgan DE (2003) Secretions of stingless bees: the Dufour glands of some
Frieseomelitta species (Apidae, Meliponinae). Apidologie 34: 359-365. doi:
10.1051/apido:2003027Pau RN, Brunet PCJ, Williams MJ (1971) The isolation and characterization of proteins from the left colleterial gland of the cockroach,
Periplaneta americana (L.). Proceedings of the Royal Society of London B 177: 565-579. doi:
10.1098/rspb.1971.0048Rasnitsyn AP (2002) Class Insecta Linné, 1758. The insects (=Scarabaeoda Laicharting, 1781). In: Rasnitsyn AP, Quicke DLJ (Eds) History of insects. Kluwer Academic Publishers, Dordrecht, Netherlands, 243. doi:
10.1007/0-306-47577-4_2Rasnitsyn AP, Zhang H (2010) Early evolution of Apocrita (Insecta, Hymenoptera) as indicated by new findings in the middle Jurassic of Daohugou, northeast China. Acta Geologica Sinica 84: 834-873. doi:
10.1111/j.1755-6724.2010.00254.xReed HC (1982) Biology and behavior of the forest yellowjacket,
Vespula acadica (Sladen) and the obligate social parasite,
Vespula austraica (Panzer) (Hymenoptera: Vespidae). PHD Thesis. Washington State University, Pullman.
Reed HC, Akre RD (1982) Morphological comparisons between the obligate social parasite,
Vespula austraica (Panzer), and its host,
Vespula acadica (Sladen) (Hymenoptera: Vespidae). Psyche 89: 183-196. doi:
10.1155/1982/52306Richard F-J, Schal C, Tarpy DR, Grozinger CM (2011) Effects of instrumental insemination and insemination quantity on Dufour’s gland chemical profiles and vitellogenin expression in honey bee queens (
Apis mellifera). Journal of Chemical Ecology 37: 1027-1036. doi:
10.1007/s10886-011-9999-zRitter FJ, Brüggemann-Rotgans IEM, Verwiel PEJ, Persoons CJ, Taiman E (1977) Trail pheromones of the Pharaoh’s ant,
Monomorium pharaonis: isolation and identification of Farnal, a terpenoid related to juvenile hormone II. Tetrahedron Letters (London) 30: 2617-2618. doi:
10.1016/S0040-4039(01)83835-1Robertson PL (1968) A morphological and functional study of the venom apparatus in representatives of some major groups of Hymenoptera. Australian Journal of Zoology 16: 133–166. doi:
10.1071/ZO9680133Rosi MC, Isidoro N, Colazza S, Bin F (2001) Source of the host marking pheromone in the egg parasitoid
Trissolcus basalis (Hymenoptera: Scelionidae). Journal of Insect Physiology 47: 989-995. doi:
10.1016/S0022-1910(01)00073-7Ruano F, Hefetz A, Lenoir A, Francke W, Tinaut A (2005) Dufour’s gland secretion as repellent used during usurpation in the slave-maker ant
Rossomyrmex minuchae. Journal of Insect Physiology 51: 1158-1164. doi:
10.1016/j.jinsphys.2005.06.005Schmitz J, Moritz RFA (1998) Molecular phylogeny of Vespidae (Hymenoptera) and the evolution of sociality in wasps. Molecular Phylogenetics and Evolution 9: 183-191. doi:
10.1006/mpev.1997.0460Shimron O, Hefetz A, Tengö J (1985) Structural and communicative functions of Dufour’s gland secretion in
Eucera palestinae (Hymenoptera: Anthophoridae). Insect Biochemistry 15: 635-638. doi:
10.1016/0020-1790(85)90126-XSimser H, Coppel HC (1980) Female-produced sex pheromone in
Brachymeria lasus and
B. intermedia (Hym.: Chalcididae). Biocontrol 25: 373-380. doi:
10.1007/BF02374700Sledge MF, Fortunato A, Turillazzi S, Francescato E, Hashim R, Moneti G, Jones GR (2000) Use of Dufour’s gland secretion in nest defence and brood nutrition by hover wasps (Hymenoptera, Stenogastrinae). Journal of Insect Physiology 46: 753-761. doi:
10.1016/S0022-1910(99)00164-XSmith BH, Carlson TG, Frazier J (1985) Identification and bioassay of macrocyclic lactone sex pheromone of the halictine bee
Lasioglossum zephyrum. Journal of Chemical Ecology 11: 1447-1456. doi:
10.1007/BF01012144Smith BH, Wenzel JW (1988) Pheromonal covariation and kinship in social bee
Lasioglossum zephyrum (Hymenoptera: Halictidae). Journal of Chemical Ecology 14: 87-94. doi:
10.1007/BF01022533Sole CL, Kryger P, Hefetz A, Katzav-Gozansky T, Crewe RM (2002) Mimicry of queen Dufour’s gland secretions by workers of
Apis mellifera scutellata and
A. m. capensis. Naturwissenschaften 89: 561-564. doi:
10.1007/s00114-002-0370-7Soro A, Ayasse M, Zobel MU, Paxton RJ (2011) Kin discriminators in the eusocial sweat bee
Lasioglossum malachurum: the reliability of cuticular and Dufour’s gland odours. Behavioral Ecology and Sociobiology 65: 641-653. doi:
10.1007/s00265-010-1066-1Stephen WP, Torchio PF (1961) Biological observation on
Emphoropsis miserabilis (Cresson) with comparative notes on other anthophorids. Annals of the Entomological Society of America 54: 687-692.
Stoka AM (1999) Phylogeny and evolution of chemical communication: an endocrine approach. Journal of Molecular Endocrinology 22: 207-225. doi:
10.1677/jme.0.0220207Strohm E, Linsenmair KE (2001) Females of the European beewolf preserve their honeybee prey against competing fungi. Ecological Entomology 26: 198-203. doi:
10.1046/j.1365-2311.2001.00300.xSugumaran M, Nellaiappan K (1990) On the latency and nature of phenoloxidase present in the left colleterial gland of the cockroach
Periplaneta americana. Archives of Insect Biochemistry and Physiology 15: 165-181. doi:
10.1002/arch.940150305Syvertsen TC, Jackson LL, Blomquist GJ, Vinson SB (1995) Alkadienes mediating courtship in the parasitoid
Cardiochiles nigriceps (Hymenoptera: Braconidae). Journal of Chemical Ecology 21: 1971-1989. doi:
10.1007/BF02033856Tengö J, Bergström G (1977) Cleptoparasitism and odor mimetism in bees: do
Nomada males imitate the odor of
Andrena females? Science 196: 1117–1119. doi:
10.1126/science.196.4294.1117Tengö J, Hefetz A, Bertsch A, Schmitt U, Lübke G, Francke W (1991) Species specificity and complexity of Dufour’s gland secretion of bumble bees. Comparative Biochemistry and Physiology 99B: 641–646. doi:
10.1016/0305-0491(91)90348-HTopoff H, Cover S, Greenberg L, Goodloe L, Sherman P (1988) Colony founding by queens of the obligatory slave-making ant,
Polyergus breviceps: the role of the Dufour’s gland. Ethology 78: 209-218. doi:
10.1111/j.1439-0310.1988.tb00231.xTraniello JFA (1980) Colony specificity in the trail pheromone of an ant. Naturwissenschaften 67: 361-362. doi:
10.1007/BF01106597Turillazzi S (1989) The origin and evolution of social life in the Stenogastrinae (Hymenoptera, Vespidae). Journal of Insect Behavior 2: 649-661. doi:
10.1007/BF01065784Ueno T (1994) Self-recognition by the parasitic wasp
Itoplectis naranyae (Hymenoptera: Ichneumonidae). Oikos 70: 333-339. doi:
10.2307/3545770Van Marle J, Piek T (1986) Morphology of the venom apparatus. In: Piek T (Ed) Venoms of the Hymenoptera. Academic Press, London, 17-44.
Vardal H (2006) Venom gland and reservoir morphology in cynipoid wasps. Arthropod Structure and Development 35: 127-136. doi:
10.1016/j.asd.2006.05.002Vincent B, Kaeslin M, Roth T, Heller M, Poulain J, Cousserans F, Schaller J, Poirie M, Lanzerin B, Drezen J-M, Moreau SJM (2010) The venom composition of the parasitic wasp
Chelonus inanitus resolved by combined expressed sequence tags analysis and proteomic approach. BMC Genetics 11: 693. doi:
10.1186/1471-2164-11-693Vinson SB (1978) Courtship behavior and source of a sexual pheromone from
Cardiochiles nigriceps. Annals of the Entomological Society of America 71: 832-837.
Vinson SB, Frankie GW, Blum MS, Wheeler JW (1978) Isolation, identification and function of Dufour’s gland secretion of
Xylocopa virginica texana (Hymenoptera: Anthophoridae). Journal of Chemical Ecology 4: 315-323. doi:
10.1007/BF00989340Vinson SB, Guillot FS (1972) Host marking: Source of a substance that results in host discrimination in insect parasitoids. Biocontrol 17: 241-245. doi:
10.1007/BF02371134Whitehouse MEA, Jaffe K (1996) Ant wars: combat strategies, territory and nest defence in the leaf-cutting ant
Atta laevigata. Animal Behaviour 51: 1207-1217. doi:
10.1006/anbe.1996.0126Whitfield JB (1992) Phylogeny of the non-Aculeate Apocrita and the evolution of parasitism in the Hymenoptera. Journal of Hymenoptera Research 1: 3-14.
Whitfield JB, Cameron SA (1998) Hierarchical analysis of variation in the mitochondrial 16S rRNA gene among Hymenoptera. Molecular Biology and Evolution 15: 1728-1743. doi:
10.1093/oxfordjournals.molbev.a025899Williams HJ, Strand MR, Vinson SB (1981) Trail pheromone of the red imported fire ant
Solenopsis invicta (Buren). Cellular and Molecular Life Sciences 37: 1159-1160. doi:
10.1007/BF01989893Wilson EO, Regnier FE Jr. (1971) The evolution of the alarm-defense system in the Formicine ants. The American Naturalist 105: 279-289. doi:
10.1086/282724