(C) 2013 John M. Heraty. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Heraty JM, Murray E (2013) The life history of Pseudometagea schwarzii, with a discussion of the evolution of endoparasitism and koinobiosis in Eucharitidae and Perilampidae (Chalcidoidea). Journal of Hymenoptera Research 35: 1–15. doi: 10.3897/JHR.35.6025
The immature stages and behavior of Pseudometagea schwarzii (Ashmead) (Hymenoptera: Eucharitidae: Eucharitini) are described, and the presence of an endoparasitic planidium that undergoes growth-feeding in the larva of the host ant (Lasius neoniger Emery) is confirmed. Bayesian inference and parsimony ancestral state reconstruction are used to map the evolution of endoparasitism across the eucharitid-perilampid clade. Endoparasitism is proposed to have evolved independently three times within Eucharitidae, including once in Pseudometagea Ashmead, and at least twice in Perilampus Latreille. Endoparasitism is independent as an evolutionary trait from other life history traits such as differences in growth and development of the first-instar larva, hypermetamorphic larval morphology, and other biological traits, including koinobiosis.
Eucharitidae, endoparasitism, koinobiosis, hyperparasitism, Formicidae
Eucharitidae and Perilampidae form a monophyletic group within Chalcidoidea (Hymenoptera) (
Development on the host pupa is nearly identical for all taxa, with the first and later instars developing as ectoparasitoids typically on the posterior ventral thoracic region of the host pupa (
Oviposition, host location and planidial behaviors vary across Eucharitidae and Perilampidae. Eggs of Chrysolampinae are deposited in the flower head or seed pod, and larvae move to the weevil host, where they remain as ectoparasitoids until host pupation (
Both Eucharitidae and Perilampidae are koinobionts, that is, parasitoids that do not kill their host initially but instead transition through more than one host life history stage (
Hypermetamorphic larvae with both discrete morphologies and behaviors across different instars is uncommon within the Hymenoptera. Type I hypermetamorphism involves oviposition away from the food source and an active first-instar parasitoid (
Both endoparasitism and koinobiosis are derived and likely independent traits within Hymenoptera (
In this paper, we confirm the observation of endoparasitism within the genus Pseudometagea, and propose that endoparasitism may have developed at least five times within the perilampid-eucharitid lineage. We use these data to interpret the evolution of endoparasitism and koinobiosis within this specialized group of type I hypermetamorphic Chalcidoidea.
A total of 46 colonies of Lasius neoniger were sampled from under stones along a roadside in the Ojibway Long Grass Prairie Nature Reserve, Windsor, Ontario, Canada (42°15'42.9"N, 83°04'02.2"W) from 2–4 June, 1982. No attempt was made to sample entire colonies, but instead only the larvae, cocoons and representative adult ants that could be readily aspirated. Collections were stored in 70% ethanol. Cocoons were dissected and the contents examined for parasitized ant larvae, prepupae, ant pupae or parasitoid pupae. Representative adults and larval stages of both Pseudometagea and Lasius were dried using hexamethyldisilazane (HMDS,
Literature records for internal or external parasitism by planidia on their larval host are known for 27 species of Perilampidae (Chrysolampinae and Perilampinae) and Eucharitidae (
Taxa used in reduced analysis, with references to the mode of parasitism and taxon substitutes (grey) from the matrix. Voucher codes and Genebank accession numbers are included. Classification abbbreviations: PEC Chrysolampinae; PEP Perilampinae; EUG Gollumiellinae; EUO Oraseminae; EUP Eucharitinae: Psilocharitini; EUE Eucharitinae: Eucharitini.
| Classif. | Reference taxon | planidial mode | Reference | Taxon in analysis | D_number | Genbank Accession Numbers | |||
|---|---|---|---|---|---|---|---|---|---|
| 18S | 28S D2-D5 | COI | COII | ||||||
| PEC | Chrysolampus sisimbryi | ectoparasite | Chrysolampus sisimbryi | D0970 | JN623326 | AY552188 | KC008309 | – | |
| PEC | Chrysolampus thenae | ectoparasite | Chrysolampus sp. (Australia) | D0160 | JN623327 | AY552185, JN624069 | – | – | |
| PEP | Steffanolampus salicetum | ectoparasite | Steffanolampus salicetum | D0320 | JN623354 | AY552177, JN624088 | KC008323 | – | |
| PEP | Monacon robertsi | ectoparasite | Monacon robertsi | D0318 | JN623340 | AY552181, JN624078 | KC008315 | – | |
| PEP | Perilampus chrysopae | ectoparasite | Chrysolampus sp. (Australia) | D0113 | JN623345 | AY552178 | KC008319 | – | |
| PEP | Perilampus fulvicornis | endoparasite | Perilampus fulvicornis | D0713 | JN623342 | JN623717, JN624080 | KC008217 | KC008489 | |
| PEP | Perilampus hyalinus | endoparasite | Perilampus hyalinus | D0972 | AY552257 | AY552180 | KC008318 | – | |
| EUG | Gollumiella longipetiolata | endoparasite | Gollumiella longipetiolata | D0405c | JN623252 | AY552191, JN624020 | KC008166 | KC008328 | |
| EUO | Orasema costaricensis | endoparasite | Orasema costaricensis | D0342 | – | AY672931 | – | – | |
| EUO | Orasema simulatrix | endoparasite | Orasema simulatrix | D0422 | JN623259 | AY552206, JN624027 | KC008181 | – | |
| EUO | Orasema sixaolae | endoparasite | Chrysolampus sp. (Australia) | D2919 | KC008506 | KC008091, KC008142 | KC008182 | – | |
| EUO | Orasema viridis | endoparasite | Chrysolampus sp. (Australia) | D0248 | – | AY672955, KC008493 | – | – | |
| EUO | Orasema xanthopus | endoparasite | Orasema xanthopus | D1090 | KC008510 | KC008093, KC008143 | – | – | |
| EUO | Timioderus acuminatus | endoparasite | Chrysolampus sp. (Australia) | D0116 | JN623266 | AY552195, JN624034 | – | KC008334 | |
| EUP | Neolosbanus palgravei | ectoparasite | Neolosbanus palgravei | D2845 | – | KC008096, KC008146 | KC008186 | KC008348 | |
| EUE | Ancylotropus manipurensis | ectoparasite | Ancylotropus cariniscutis (Malaysia) | D0701 | – | KC008109 | KC008233 | KC008412 | |
| EUE | Austeucharis fasciiventris | ectoparasite | Austeucharis sp. (Australia: NSW) | D0904 | – | AY671806 | KC008220 | KC008398 | |
| EUE | Chalcura affinis | ectoparasite | Clausen 1940 | Chalcura sp. (Australia: NT) | D0647 | – | KC008099 | KC008211 | KC008387 |
| EUE | Dicoelothorax platycerus | ectoparasite | Dicoelothorax platycerus | D2512 | – | KC008115, KC008149 | – | KC008431 | |
| EUE | Eucharis adscendens | ectoparasite | Clausen 1940 | Eucharis adscendens | D0729 | JN623231 | AY552229, JN624007 | KC008189 | KC008353 |
| EUE | Galearia bruchii | ectoparasite | Galearia bruchii | D2491 | KC008531 | KC008117 | – | – | |
| EUE | Kapala terminalis | ectoparasite | Clausen 1940 | Kapala terminalis | D1270 | KC008561 | AY671891 | KC008290 | KC008479 |
| EUE | Latina rugulosa | ectoparasite | Latina guriana (Argentina: SA) | D1073b | AY552319 | AY552242 | KC008246 | KC008433 | |
| EUE | Pseudochalcura gibbosa | ectoparasite | Pseudochalcura gibbosa | D0910 | AY552295 | AY552218 | KC008199 | KC008373 | |
| EUE | Pseudometagea schwarzii | endoparasite | Pseudometagea schwarzii | D0274 | AY552292 | AY552215 | KC008188 | KC008352 | |
| EUE | Stilbula cyniformis | ectoparasite | Clausen 1940 | Stilbula sp. 1 (Nigeria) | D2692 | AY552301 | GQ331923, KC008495 | – | KC008367 |
| EUE | Stilbula tenuicornis | ectoparasite | Stilbula sp. 2 (Singapore) | D2837 | KC008517 | KC008097 | KC008196 | KC008368 | |
Exemplar trees for trait mapping were pruned from the Bayesian analysis of molecular data (18S, 28S, COI, COII) for 237 taxa by
During analysis, BayesTraits picks one of the 10, 000 trees randomly for each iteration and calculates state probabilities at designated nodes of interest. There are two nodes that are not present on all trees: Perilampinae + Eucharitidae, and Eucharitini excluding Pseudometagea. For trees without the node present, the probability of the ancestral states was set as uninformative at a 0.5 probability for each state. This incorporates uncertainty of the node in proportion to its appearance in the distribution of trees. Tracer v1.5.0 (
Mesquite v2.73 (
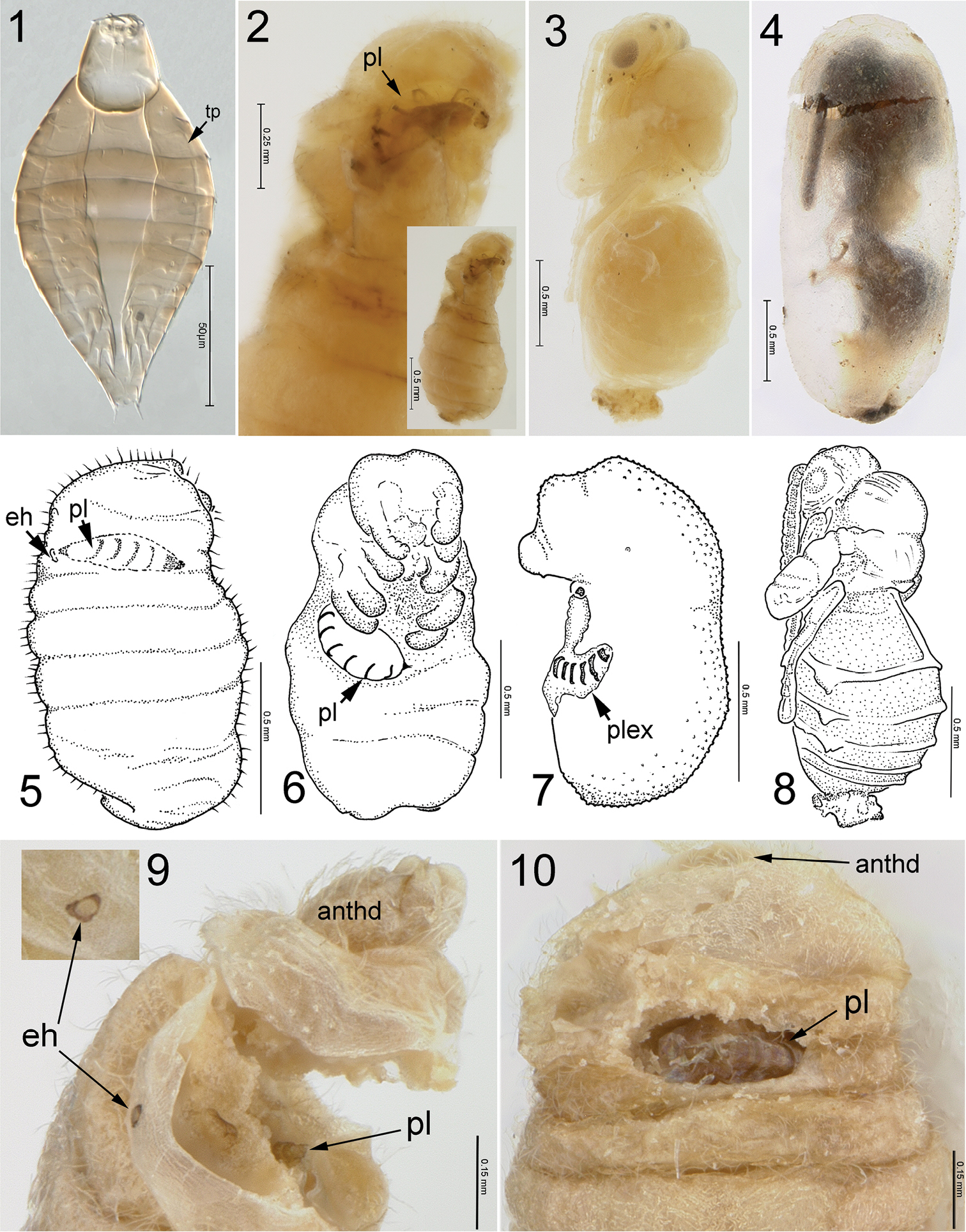
Fourteen (30.4%) of the 47 colonies of Lasius neoniger sampled were parasitized by Pseudometagea schwarzii. From these parasitized colonies, we recovered 17 unparasitized mature ant larvae, 473 unparasitized ant pupae, and 115 parasitized cocoons. Of the parasitized cocoons, there were 15 mature ant larvae within the cocoon with an internal planidium of Pseudometagea schwarzii (PS), 7 ant pupae with external PS planidia, 5 ant pupae with a second instar PS, 2 ant pupae with a third instar PS, and86 PS pupae. Only one parasitoid per host larva or pupa was ever observed. Three larval instars were observed for Pseudometagea schwarzii. The few mature larvae and numerous ant and parasitoid pupae suggest that sampling took place at the end of the overwintering generation of brood for that year. Numerous males of Pseudometagea schwarzii flying over the ant colonies during collection also suggested a recent emergence. One female emerging from the nest entrance attracted several males that swarmed the female in a ball on the ground. The supposedly now mated female left the cluster of males and flew to the nearest host plant and began oviposition into flower heads of Erigeron strigosus Muhl. ex Willd. (Asteraceae).
The unfed planidium (Fig. 1), taken from these same collections, was described by
Pseudometagea schwarzii. 1 planidium (first-instar), tp = tergopleural line 2 Lasius neoniger larva with internal planidium (pl), inset is full size ant larva (from cocoon) 3 female pupa 4 adult male in process of opening cocoon 5 Lasius larva with planidium, eh= entrance hole 6 Lasius pupa with external planidium 7 third instar Pseudometagea with attached planidial exuvium (plex) 8 Pseudometagea pupa 9 ant larva with entrance hole and broken to show internal planidium (apical segments), anthd = ant head 10 ant larva with dissection showing fed (expanded) internal planidium.
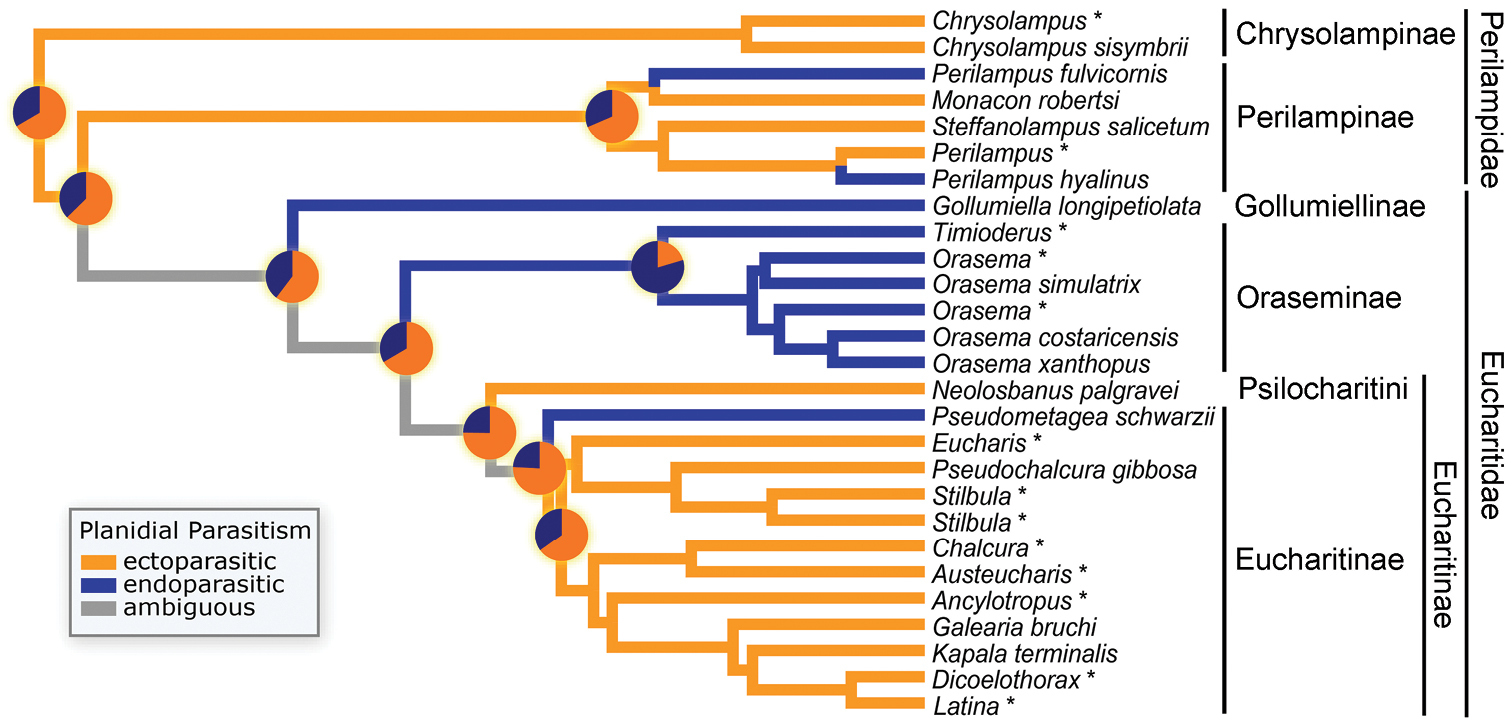
Ancestral states reconstruction for ectoparasitism and endoparasitism was done using BayesTraits (Bayesian inference) and Mesquite (parsimony) (Fig. 1). Relationships and branch lengths were based on
Ancestral character reconstruction of mode of planidial parasitism. Terminal taxa coded as either ectoparasitic (orange) or endoparasitic (blue). Branches of phylogeny colored according to parsimony reconstruction, with gray branches indicating ambiguity. Pie charts at selected nodes show probabilities of each state from the Bayesian analysis. Taxa were coded directly from life history records except where indicated by asterisks, when taxa were substituted for those included in the analyses of
Three types of feeding by planidia have been observed on the host larva. The planidia of Perilampidae are all considered as non-feeding (little to no expansion of body segments) while on or in the host larvae (
Our results confirm the report by
One of the important discoveries noted by
The causes for developing an endoparasitic lifestyle in these taxa are unclear. For most koinobionts, endoparasitism avoids issues associated with detachment during host moults between instars. However, the majority of both Eucharitidae and Perilampidae are ectoparasitic during all life stages, and retain enough mobility to relocate and reattach to the host after each moult. In Perilampidae, endoparasitism is associated with hyperparasitism, with planidia entering the primary host and then locating and attacking the ichneumonid or tachinid parasitoid within (
Potentially, endoparasitism could be associated with overwintering in the ant larval host.
Overwintering in the egg stage is known for some Eucharitinae (
Pseudometagea occupy a phylogenetically important position within the family as the sister group of the remaining Eucharitini (Fig. 11). Their planidia are endoparasitic and overwinter on the larvae of their host ant, and they have a growth phase of development while in the larval host. Endoparasitism in Perilampidae may be correlated with hyperparasitism. Within Eucharitidae, our results suggest that endoparasitism has developed multiple times (Fig. 11), with its appearance independent of other derived larval behaviors including growth feeding, association with ants that have a cocoon, and overwintering. The reasons for the derivation of each of these traits are unclear, and future studies of both eucharitid and ant biology are necessary to better understand the evolution of parasitoid developmental behaviors.
We would like to thank Kevin Barber for alerting JMH to the Ojibway collection site. Helpful comments on drafts of this manuscript were provided by Chris Darling (Royal Ontario Museum) and John Hash and Jason Mottern (UCR). This research was funded in part by NSF DEB 0730616 and 1257733 to JMH.