Citation: Fernández-Triana J, Shaw MR, Cardinal S, Mason PG (2014) Contributions to the study of the Holarctic fauna of Microgastrinae (Hymenoptera, Braconidae). I. Introduction and first results of transatlantic comparisons. Journal of Hymenoptera Research 37: 61–76. doi: 10.3897/JHR.37.7186
Specimens of Microgastrinae (Hymenoptera: Braconidae) from both sides of the Holarctic region (Nearctic and Palaearctic) were sampled for DNA barcoding and examined morphologically. Two species are recorded for the first time for the Nearctic: Apanteles brunnistigma Abdinbekova, and Microgaster raschkiellae Shaw. Another European species, Apanteles xanthostigma (Haliday), previously introduced as a biological control agent, is confirmed to be present in North America. For another 13 species significant range extension is documented, including new records for France, Canada, United States, and Sweden. New host data are also provided for several species. The species name Apanteles masmithi Fernández-Triana is considered a syn. n. of Dolichogenidea britannica (Wilkinson).
Microgastrinae, DNA barcoding, morphology, Holarctic, Palaearctic, Nearctic
Microgastrinae wasps (Hymenoptera: Braconidae) are one of the most important groups in the biological control of Lepidoptera caterpillar pests of forestry and agriculture (
According to the latest version of Taxapad (
Altogether, the Nearctic plus the Palaearctic (i.e., the Holarctic region) have 1029 species, accounting for 46% of the described microgastrines worldwide. This, of course, is an artifact due to most studies being historically focused on the north-temperate areas of the planet. There is a significant diversity waiting to be described in the tropics, totalling several thousand new species (e.g.,
While the Palaearctic has twice the number of known species as the Nearctic, the final figures might be much closer. Most of the European (i.e. Western Palaearctic) species are already known, with the remaining diversity to be discovered being mostly morphologically cryptic species. On the other hand, the Eastern Palaearctic is much less studied, and it is likely that a significant number of additional species remain to be found and described there, although we are not aware of any published information providing estimates. The most updated list of species for the Palaearctic can be found in Fauna Europaea (van
As for the Nearctic region, it is clear that a considerable number of species are still unknown. For example,
According to the published data, only 272 (26%) of the 1, 029 Holarctic species of Microgastrinae are shared between the Nearctic and Palaearctic regions. This relatively low percentage might be due to some taxa being described twice, as different species, on the two sides of the Atlantic. The large number of descriptions, and the holotypes being scattered across a large number of collections, make it very difficult to approach the study of this group from a truly Holarctic perspective. Thus, taxonomic revisions of Microgastrinae have usually focused on either the Palaearctic or the Nearctic, with very few studies covering both regions.
DNA barcoding uses a short standardized region of the mitochondrial gene cytochrome c oxidase (COI) as a key character for species-level identification and discovery (
This paper is mostly based in the study of DNA barcoded specimens of Microgastrinae from the Canadian National Collection, Ottawa (CNC), and the National Museums of Scotland, Edinburgh (NMS). Additionally, some barcoded specimens from the Swedish Malaise Trap Project (SMTP) were available for study; the SMTP aims to provide species determinations for all the 80 million insect specimens obtained from Malaise traps sampling at a wide range of landscapes and habitats in Sweden (http://www.stationlinne.se/en/research/the-swedish-malaise-trap-project-smtp/). Pictures of barcoded specimens housed in the Biodiversity Institute of Ontario, Guelph were also analyzed. Examination of holotypes deposited in the National Museum of Natural History, Washington D.C. (NMNH), and the Natural History Museum, London (BMNH), was made in some cases – mostly when necessary to verify identifications of specimens in the CNC and NMS.
One of us (MRS) provided samples of reliably determined Microgastrinae from Europe for barcoding, reared from identified hosts that are in many cases the very hosts from which the species in question was originally described. This provides a framework of fixed reference points from which to assess the specific identity of other specimens, whether reared or not and from both the Nearctic and the Palaearctic, initially through their barcodes. When host names are given for specimens in NMS, we append “Det.” and then the name of the person who reared the caterpillars, because it was (s)he who was responsible for the host determination.
To uncover new distribution patterns, we first scanned the tree presented in
Newly generated DNA barcodes were obtained using DNA extracts prepared from single legs using a glass fibre protocol (
Details of specimens from the National Museums of Scotland (NMS) with DNA barcode sequences newly obtained – and not yet available in the Barcode of Life Data Systems (BOLD). Collection codes for NMS and the Canadian National Collection of Insects (CNC), as well as GenBank accession numbers are included.
| Wasp species | Host species | Locality | Emergence date | NMS code | CNC code | GenBank accession numbers |
|---|---|---|---|---|---|---|
| Apanteles brunnistigma | Pyrausta aurata | France: Aveyron, Livinhac-le-Haut | 18.vi.2012 | MRS 0183 | CNCHYM49298 | KJ459123 |
| Apanteles brunnistigma | Pyrausta aurata | France: Aveyron, Livinhac-le-Haut | 11.vi.2012 | MRS 0184 | CNCHYM49319 | KJ459223 |
| Dolichogenidea britannica | Ptocheuusa paupella | England: Hants, Portsmouth | vii.2011 | MRS 0217 | CNCHYM45321 | KJ459126 |
| Microgaster raschkiellae | Mompha raschkiella | Scotland: Skye, Armadale | 4.vii.2012 | MRS 0192 | CNCHYM45380 | KJ459161 |
| Pholetesor viminetorum | Elachista poae | England: W. Yorks, Copley | 28.iv.2012 | MRS 0166 | CNCHYM45332 | KJ459179 |
Sequences were considered as “barcode-compliant” when they had 500 or more base pairs and had less than 1% ambiguous characters (Barcode Compliance standards as in http://www.boldsystems.org/index.php/resources/handbook?chapter=6_managingdata.html§ion=record_list).
Genera (and species within a genus) are presented in alphabetical order. Each new distribution record is discussed within the context of the previously known distribution of the species. For Canadian provinces and territories, and states of the United States we use acronyms consisting of two capital letters following Canada Post (http://www.canadapost.ca/tools/pg/manual/PGaddress-e.asp).
Photos were taken with a Keyence VHX-1000 Digital Microscope, using a lens with a magnification range of 13–130×. Multiple images through the focal plane were taken of a structure and these were combined to produce a single in-focus image, using the software associated with the Keyence System.
Two species previously known only from the Palaearctic are here recorded for the first time for the Nearctic. Another European species, previously introduced as a biological control agent but not known to be established, is here confirmed to be still present in North America. Additionally, another 13 species have been found to have a wider range than previously known – in some cases the new data reported here significantly expand the known distributions.
Most of the range expansions recorded were towards northern areas (e.g. Alaska, Manitoba, Nunavut, and Yukon Territory) and may reflect an increase in the availability of material recently collected in arctic and sub-arctic localities of North America (e.g.,
Figs 1–4
Previously, this species was known to be widely distributed in the Palaearctic (
Apanteles brunnistigma had been previously recorded as a parasitoid of Depressariidae (Agonopterix umbellana (Fabricius), Depressaria ultimella Stainton), and Tortricidae (Aphelia viburnana (Denis & Schiffermüller), Archips rosana (Linnaeus), Eucosma rubescana (Constant) (as catoptrana), and Gynnidomorpha vectisana (Humphries and Westwood)), as summarized in
We analyzed 61 barcode sequences available for this species (59 of them barcode compliant), representing 11 haplotypes from Canada, France, Sweden, and Ukraine. The difference between haplotypes ranged from one to 10 base pairs (0.2–1.5%), with most sequences differing by five base pairs or less.
Apanteles brunnistigma. 1 Habitus, lateral view, Swedish specimen with CNC code CNCH1798 2 Habitus, lateral view, Canadian specimen with CNC code 07PROBE-23429 3 Head, mesosoma and mediotergites 1-2, dorsal view, specimen CNCH1798 4 Metasoma, dorsal view, specimen CNCH1798.
This species was known to be widely distributed in the Nearctic (
This species had been previously recorded from the Lepidoptera families Tortricidae (Choristoneura occidentalis Freeman), and Crambidae (Fissicrambus mutabilis (Clemens), and Neodactria zeella (Fernald)), as summarized in
We analyzed 178 barcode sequences available for this species (166 of them barcode compliant), representing 20 haplotypes from Canada and the United States. The difference between haplotypes ranged from one to eight base pairs (0.2–1.2%).
Figs 5–7
Previously, the species was known to be widely distributed in the Palaearctic (
In the NMS there are authenticated specimens reared from the following Lepidoptera families and species: Choreutidae (Choreutis diana (Hübner) (R. J. Heckford)), Gracillariidae (Caloptilia betulicola (Hering) (Det. S. D. Beavan, K. P. Bland, R. J. Heckford, J. R. Langmaid, M. R. Shaw, P. A. Sokoloff), Caloptilia elongella (Linnaeus) (Det. K. P. Bland, M. R. Shaw), Caloptilia stigmatella (Fabricius) (Det. P. J. Johnson, J. R. Langmaid, S. E. Whitebread), Povolnya leucapennella (Stephens) (Det. R. J. Heckford) new host record, Parornix devoniella (Stainton) (Det. M. R. Shaw) new host record, and Parornix scoticella (Stainton) (Det. M. R. Shaw) new host record), Pyralidae (Acrobasis suavella (Zincken) (Det. M. F. V. Corley, R. J. Heckford) new host record), Tortricidae (Acleris hastiana (Linnaeus) (Det. P. J. Johnson) new host record, Adoxophyes orana (Fischer von Röslerstamm) (Det. A. Wilson), Rhopobota naevana (Hübner) (Det. M. R. Young)), and Yponomeutidae (Swammerdamia caesiella (Hübner) (Det. M. R. Shaw), Swammerdamia pyrella (Villers) (Det. J. L. Gregory), Paraswammerdamia albicapitella (Scharfenberg) (Det. J. L. Gregory, N. Hall)).
We analyzed 12 barcode sequences available for this species (10 of them barcode compliant), representing four haplotypes from Canada, the Netherlands, Russia, and Sweden. The difference between haplotypes ranged from one to eight base pairs (0.2–1.2%), with most sequences differing by six base pairs or less.
The fact that new Canadian records come from several localities, all far apart (more than 3, 000 km) from the original site of introduction in Newfoundland, suggests that the species was probably already established in North America prior to the 1963 introduction.
Apanteles xanthostigma. 5 Habitus, dorsal view, Swedish specimen with CNC code CNCH1804 6 Head and mesosoma, dorsal view, specimen CNCH1804 7 Propodeum and metasoma, dorsal view, specimen CNCH1804.
This species was previously known from northeast and central United States (
We analyzed six barcode sequences available for this species (two of them barcode compliant), representing 3 haplotypes from Canada and the United States. The difference between haplotypes was one base pair (0.2%).
Previous records show a wide distribution within the Nearctic, although records are scarce and sparse over North America. Here it is recorded for the first time in three additional Canadian provinces/territories (AB, Waterton Lakes National Park, one specimen; MB, Churchill, four specimens; and YT, Top of the World Highway, km 82, one specimen) and one state of the United States (AK, Anchorage, three specimens). The new records expand northward the known distribution of the species. Some of the specimens from Manitoba were named as “Cotesia jft04” in a previous paper (
We analyzed 16 barcode sequences available for this species (seven of them barcode compliant), representing five haplotypes from Canada. The difference between haplotypes ranged from one to 13 base pairs (0.2–2.0%).
This species was previously known from eastern and central United States and the Canadian province of Nova Scotia. Here it is recorded for the first time for two additional states of the United States (NJ, Metuchen, one specimen; and AL, Mobile County, Country Road 1, one specimen). Five species of Lepidoptera, one in Lasiocampidae and four in Noctuidae, have been recorded as hosts of the species (
We analyzed six barcode sequences available for this species (two of them barcode compliant), representing 3 haplotypes from Canada and the United States. The difference between haplotypes ranged from one to three base pairs (0.2–0.5%).
This species was previously known from Belgium and Finland. Here recorded for the first time for Sweden (Småland, Nybro kommun, Bäckebo, Grytsjöns naturreservat, Lat/Lon: 63.1766, 15.3005, one specimen from the SMTP).
The reared material (the type series) in NMS is from Boloria selene (Dennis & Schiffermüller) (Lepidoptera: Nymphalidae) (C. Turlure, J. Choutt), so far the only known host of Cotesia selenevora. There are no records additional to those given by Shaw (2009).
We analyzed two barcode sequences available for this species (both barcode compliant), representing 1 haplotype from Belgium and Sweden.
Apanteles masmithi Fernández-Triana, 2010. Syn. n.
After comparing the type material of Dolichogenidea britannica and Apanteles masmithi, we conclude that both are the same species – and thus the latter name becomes a synonym of the first. This represents the first record of Dolichogenidea britannica for the Nearctic. The evidence from morphology is also supported by DNA barcoding – which indeed had suggested the synonymy in the first place. The difference was only three base pairs (0.5%) between the two available haplotypes (one haplotype from eight Canadian specimens (three specimens barcode compliant), the other haplotype from one English specimen (barcode compliant) reared from the same host species as the type). This species is an example of the need to study the Holarctic fauna as a whole, whenever possible; otherwise the same species is likely to be described twice (or more) on both sides of the Atlantic.
The type series of Dolichogenidea britannica was reared from the gelechiid now known as Ptocheuusa paupella (Zeller). An additional host record given by
This species was previously known in the Nearctic from three rather separate areas (CT in the United States, BC and PE in Canada). Here it is recorded for the first time, from numerous specimens, from six additional provinces/territories in Canada (AB, Banff National Park; MB, 22 km S of Camperville; Churchill; 1 km N of Winnipeg; NL, Corner Brook; Port Saunders; St-Andrew’s; QC, Belle-Anse; SK, Grasslands National Park; and YT, Whitehorse); and one state in the United States (AK, S of Anchorage). The new records show the species is rather widely distributed within the Nearctic north of 40°N. Specimens were previously named as Dolichogenidea jft09 in
We analyzed 70 barcode sequences available for this species (64 of them barcode compliant), representing 12 haplotypes from Canada and the United States. The difference between haplotypes ranged between one and three base pairs (0.2–0.5%).
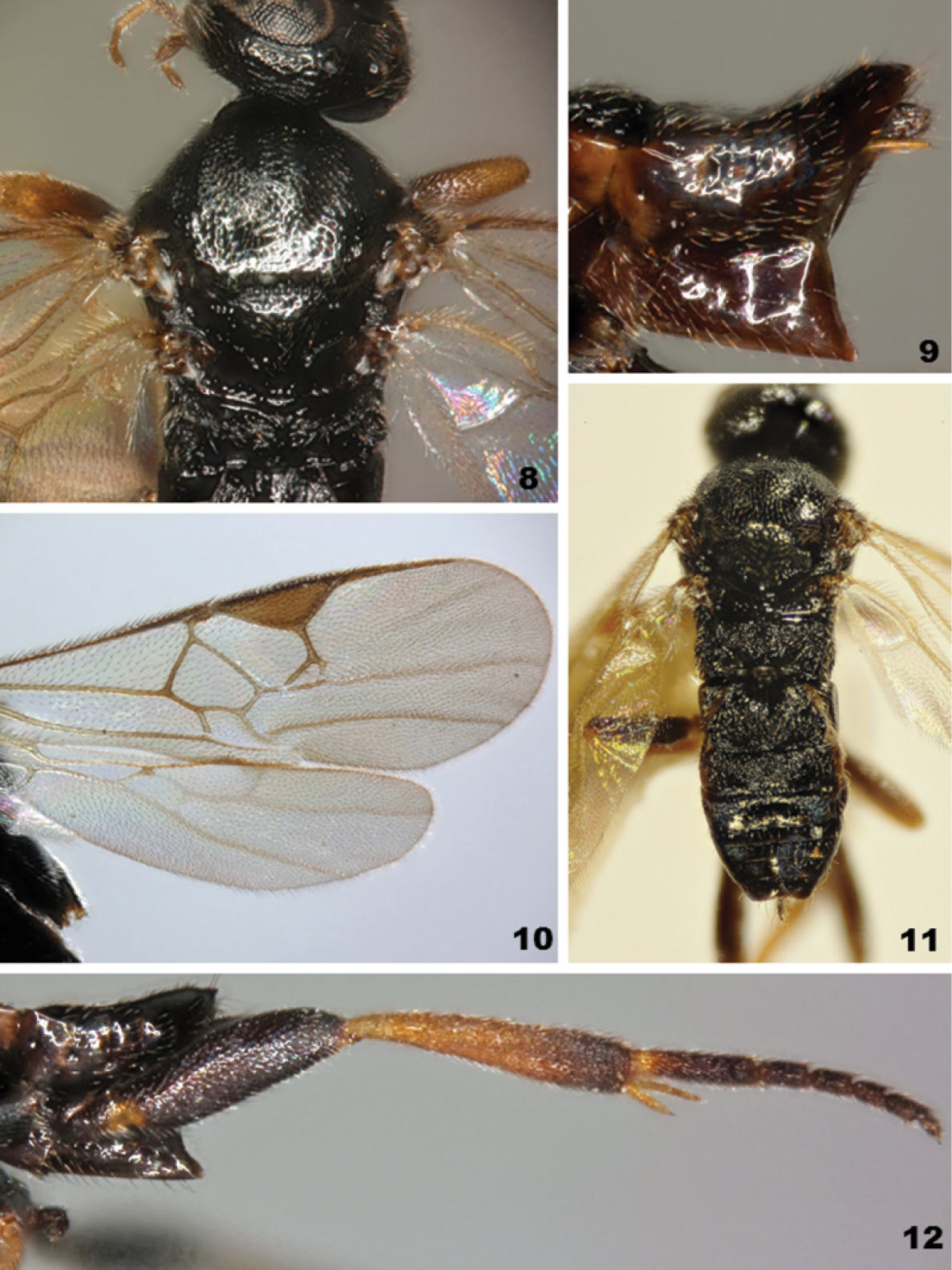
Figs 8–12
This species was recently described from Great Britain (England, Scotland and Wales), where it was widely recovered as the commonest parasitoid of Mompha raschkiella (Zeller) (Lepidoptera: Momphidae). Because it has not been reared from any other species of Mompha,
This species was described too late for inclusion in
Microgaster raschkiellae (all photos from specimens from the United Kingdom part of the original description of the species) 8 Mesoscutum and scutellum 9 Metasoma in lateral view 10 Wings 11 Habitus, dorsal view 12 Hind leg (excluding coxa).
This species is widely distributed within the Nearctic. Here it is recorded for the first time in three additional Canadian provinces (NL, Corner Brook; NS, Kentville Research Station; Cape Breton Highlands National Park, MacIntosh Brook; and PEI, Harrington), and one state in the United States (NJ, Metuchen). Three species of Noctuidae have been recorded as hosts of the species (
We analyzed 38 barcode sequences available for this species (35 of them barcode compliant), representing 1 haplotype from Canada.
This species is widely distributed within the Nearctic, and is also reported from Finland (
We analyzed 66 barcode sequences available for this species (60 of them barcode compliant), representing seven haplotypes from Canada and the United States. The difference between haplotypes ranged between one and five base pairs (0.2–0.8%).
This species is widely distributed within the Nearctic, and also reported from Chiapas, Mexico (
We analyzed eight barcode sequences available for this species (seven of them barcode compliant), representing four haplotypes from Canada. The difference between haplotypes ranged between one and seven base pairs (0.2–1.0%).
Previously known records for this species show a wide distribution within the Nearctic, although records are scarce and sparse over North America. Here it is recorded for the first time from two additional provinces in Canada (AB, Banff National Park, one specimen; and NL, Gros Morne National Park, Western Brook Pond, Hiking Trial, one specimen). No host is known for this species.
We analyzed three barcode sequences available for this species (two of them barcode compliant), representing one haplotype from Canada.
This species is widely distributed within the Nearctic. Here it is recorded for the first time from two additional provinces in Canada (YT, Champagne; Takhini River road; and NT, Inuvik), and one province in the United States (AK, King Salmon, Naknek River; Nome). The new records expand the known distribution of the species northward. Around 16 host species of Gracillaridae and one species in Elachistidae (Lepidoptera) have been recorded as hosts of the species (
We analyzed 40 barcode sequences available for this species (39 of them barcode compliant), representing 13 haplotypes from Canada and the United States. The difference between haplotypes ranged between one and eight base pairs (0.2–1.2%).
This species is widely distributed in the Holarctic (
We analyzed 85 barcode sequences available for this species (76 of them barcode compliant), representing 28 haplotypes from Canada. The difference between haplotypes ranged between one and 12 base pairs (0.2–1.9%).
Teresa Martin did the lab work required to obtain the new sequence data. Agriculture and Agri-Food Canada provided funding for the sequencing of some specimens from the CNC and NMS. MRS is grateful to all those who donate reared specimens to him for the NMS collection. Jeremy deWaard, Jayme Sones, Paul Hebert, and Alex Smith kindly allowed JFT to access sequences and examine photographs of specimens collected by and deposited in the Biodiversity Institute of Ontario. Pelle Magnusson and his colleagues at the Swedish Museum of Natural History in Stockholm and the Station Linné in Öland (Sweden) sent specimens of Microgastrinae collected as part of the Swedish Malaise Trap Project. The reviews of Julia Sigenberg and an anonymous reviewer improved the final version of the manuscript.